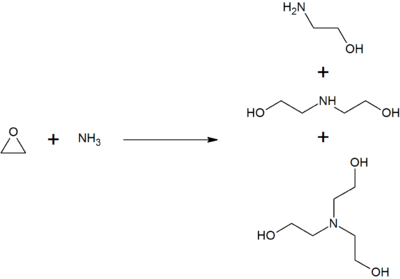Ethanolamine

| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
2-Aminoethan-1-ol[1] | |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.004.986 |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| Properties | |
| C2H7NO | |
| Molar mass | 61.084 g·mol−1 |
| Appearance | Viscous colourless liquid |
| Odor | Unpleasant ammonia-like odour |
| Density | 1.0117 g/cm3 |
| Melting point | 10.3 °C (50.5 °F; 283.4 K) |
| Boiling point | 170 °C (338 °F; 443 K) |
| Miscible | |
| Vapor pressure | 64 Pa (20 °C)[2] |
| Acidity (pKa) | 9.50[3] |
Refractive index (nD)
|
1.4539 (20 °C)[4] |
| Hazards | |
| Safety data sheet | Sigma[5] |
| GHS pictograms |  
|
| GHS Signal word | Danger |
GHS hazard statements
|
H302, H312, H332, H314, H335, H412[5] |
| P261, P273, P305+351+338, P303+361+353[5] | |
| NFPA 704 (fire diamond) | 
3
2
0 |
| Flash point | 85 °C (185 °F; 358 K) (closed cup) |
| 410 °C (770 °F; 683 K) | |
| Explosive limits | 5.5–17% |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
|
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
TWA: 3 ppm (6 mg/m3)[6] |
REL (Recommended)
|
|
IDLH (Immediate danger)
|
30 ppm[6] |
| Related compounds | |
Related compounds
|
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Ethanolamine (2-aminoethanol, monoethanolamine, ETA, or MEA) is an organic chemical compound with the formula HOCH2CH2NH2 or C2H7NO.[8] The molecule is bifunctional, containing both a primary amine and a primary alcohol. Ethanolamine is a colorless, viscous liquid with an odor reminiscent of ammonia.[9] ETA molecules are a component in the formation of cellular membranes and are thus a molecular building block for life. It was thought to exist only on Earth and on certain asteroids, but in 2021 evidence was found that ETA molecules exist in interstellar space.[10]
Derivatives of ethanolamine are widespread in nature; e.g., lipids, as precursor of a variety of N-acylethanolamines (NAEs), that modulate several animal and plant physiological processes such as seed germination, plant–pathogen interactions, chloroplast development and flowering,[11] as well as precursor, combined with arachidonic acid (C20H32O2; 20:4, ω-6), to form the endocannabinoid anandamide (AEA: C22H37NO2; 20:4, ω-6).[12]
The ethanolamines comprise a group of amino alcohols. A class of antihistamines is identified as ethanolamines, which includes carbinoxamine, clemastine, dimenhydrinate, Chlorphenoxamine, diphenhydramine and doxylamine.[13]
Industrial production[]
Monoethanolamine is produced by treating ethylene oxide with aqueous ammonia; the reaction also produces diethanolamine and triethanolamine. The ratio of the products can be controlled by the stoichiometry of the reactants.[14]
Biochemistry[]
Ethanolamine is biosynthesized by decarboxylation of serine:[15]
- HOCH2CH(CO2H)NH2 → HOCH2CH2NH2 + CO2
Ethanolamine is the second-most-abundant head group for phospholipids, substances found in biological membranes (particularly those of prokaryotes); e.g., phosphatidylethanolamine. It is also used in messenger molecules such as palmitoylethanolamide, which has an effect on CB1 receptors.[16]
Applications[]
Ethanolamine is commonly called monoethanolamine or MEA in order to be distinguished from diethanolamine (DEA) and triethanolamine (TEA). It is used as feedstock in the production of detergents, emulsifiers, polishes, pharmaceuticals, corrosion inhibitors, and chemical intermediates.[9]
For example, reacting ethanolamine with ammonia gives ethylenediamine, a precursor of the commonly used chelating agent, EDTA.[14]
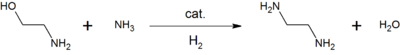
Gas stream scrubbing[]
Monoethanolamines can scrub combusted-coal, combusted-methane and combusted-biogas flue emissions of carbon dioxide (CO2) very efficiently. Monoethanolamine scrubbing reduces climate change and can make historical coal and biogas industry more modern, healthier and more marketable. Legally, it is especially relevant to the Paris Agreement. MEA carbon dioxide scrubbing is also used to regenerate the air on submarines.
Solutions of MEA in water are used as a gas stream scrubbing liquid in amine treaters. For example, aqueous MEA is used to remove carbon dioxide (CO2) and hydrogen sulfide (H2S) from various gas streams; e.g., flue gas and sour natural gas.[17] The MEA ionizes dissolved acidic compounds, making them polar and considerably more soluble.
MEA scrubbing solutions can be recycled through a regeneration unit. When heated, MEA, being a rather weak base, will release dissolved H2S or CO2 gas resulting in a pure MEA solution.[14][18]
Other uses[]
In pharmaceutical formulations, MEA is used primarily for buffering or preparation of emulsions. MEA can be used as pH regulator in cosmetics.[19]
It is an injectable sclerosant as a treatment option of symptomatic hemorrhoids. 2–5 ml of ethanolamine oleate can be injected into the mucosa just above the hemorrhoids to cause ulceration and mucosal fixation thus preventing hemorrhoids from descending out of the anal canal.
It is also an ingredient in cleaning fluid for automobile windshields. [20]
pH-control amine[]
Ethanolamine is often used for alkalinization of water in steam cycles of power plants, including nuclear power plants with pressurized water reactors. This alkalinization is performed to control corrosion of metal components. ETA (or sometimes a similar organic amine; e.g., morpholine) is selected because it does not accumulate in steam generators (boilers) and crevices due to its volatility, but rather distributes relatively uniformly throughout the entire steam cycle. In such application, ETA is a key ingredient of so-called "all-volatile treatment" of water (AVT).[citation needed]
Reactions[]
Upon reaction with carbon dioxide, 2 equivalents of ethanolamine react through the intermediacy of carbonic acid to form a carbamate salt,[21] which when heated reforms ethanolamine and carbon dioxide.
References[]
- ^ Jump up to: a b Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. pp. 649, 717. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
For example, the name ‘ethanolamine’, which is still widely used, is badly constructed because of the presence of two suffixes; it is not an alternative to the preferred IUPAC name, ‘2-aminoethan-1-ol’.
- ^ "Ethanolamine MSDS" (PDF). . Archived from the original (PDF) on 2011-07-15.
- ^ Hall, H.K., J. Am. Chem. Soc., 1957, 79, 5441.
- ^ R. E. Reitmeier; V. Sivertz; H. V. Tartar (1940). "Some Properties of Monoethanolamine and its Aqueous Solutions". Journal of the American Chemical Society. 62 (8): 1943–1944. doi:10.1021/ja01865a009.
- ^ Jump up to: a b c Sigma-Aldrich Co., Ethanolamine. Retrieved on 2018-05-24.
- ^ Jump up to: a b c NIOSH Pocket Guide to Chemical Hazards. "#0256". National Institute for Occupational Safety and Health (NIOSH).
- ^ "Ethanolamine". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- ^ "National Library of Medicine. PubChem. Ethanolomine". NIH, National Library of Medicine. Retrieved September 5, 2021.
- ^ Jump up to: a b Matthias Frauenkron, Johann-Peter Melder, Günther Ruider, Roland Rossbacher, Hartmut Höke (2002). "Ethanolamines and Propanolamines". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a10_001. ISBN 3527306730.CS1 maint: uses authors parameter (link)
- ^ "First evidence of cell membrane molecules in space". Astronomy Magazine. May 28, 2021. Retrieved September 4, 2021.
- ^ Coutinho, Bruna G.; Mevers, Emily; Schaefer, Amy L.; Pelletier, Dale A.; Harwood, Caroline S.; Clardy, Jon; Greenberg, E. Peter (2018-09-25). "A plant-responsive bacterial-signaling system senses an ethanolamine derivative". Proceedings of the National Academy of Sciences of the United States of America. 115 (39): 9785–9790. doi:10.1073/pnas.1809611115. ISSN 0027-8424. PMC 6166808. PMID 30190434.
- ^ Marzo, V. Di; Petrocellis, L. De; Sepe, N.; Buono, A. (1996-06-15). "Biosynthesis of anandamide and related acylethanolamides in mouse J774 macrophages and N18 neuroblastoma cells". Biochemical Journal. 316 (Pt 3): 977–84. doi:10.1042/bj3160977. PMC 1217444. PMID 8670178.
- ^ Cough, Cold, and Allergy Preparation Toxicity at eMedicine
- ^ Jump up to: a b c Klaus Weissermel; Hans-Jürgen Arpe; Charlet R. Lindley; Stephen Hawkins (2003). "Chap. 7. Oxidation Products of Ethylene". Industrial Organic Chemistry. Wiley-VCH. pp. 159–161. ISBN 3-527-30578-5.
- ^ "Archived copy". Archived from the original on 2012-08-21. Retrieved 2015-08-09.CS1 maint: archived copy as title (link)
- ^ Calignano, A; La Rana, G; Piomelli, D (2001). "Antinociceptive activity of the endogenous fatty acid amide, palmitylethanolamide". European Journal of Pharmacology. 419 (2–3): 191–8. doi:10.1016/S0014-2999(01)00988-8. PMID 11426841.
- ^ Emergency and Continuous Exposure Guidance Levels for Selected Submarine Contaminants. 2007. doi:10.17226/11170. ISBN 978-0-309-09225-8.
- ^ "Ethanolamine". Occupational Safety & Health Administration. Archived from the original on 2013-05-03. Retrieved 2008-05-11.
- ^ F. Carrasco (2009). "Ingredientes Cosméticos". Diccionario de Ingredientes Cosméticos 4ª Ed. www.imagenpersonal.net. p. 306. ISBN 978-84-613-4979-1.
- ^ Federal Motor Vehicle Safety Standards
- ^ Lu, Yanyue (26 December 2013). "Absorption of Carbon Dioxide in Ethanolamine Solutions". www.asianjournalofchemistry.co.in. Retrieved 2021-03-16.
External links[]
- Primary alcohols
- Amines
- Commodity chemicals
- Ethanolamines