Carbonic acid
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Carbonic acid[1]
| |||
| Other names
Hydroxyformic acid
Hydroxymethanoic acid Dihydroxycarbonyl | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
| ECHA InfoCard | 100.133.015 | ||
| EC Number |
| ||
| 25554 | |||
| KEGG | |||
PubChem CID
|
|||
CompTox Dashboard (EPA)
|
|||
| Properties | |||
| H2CO3 | |||
| Melting point | −80 °C (−112 °F; 193 K) (decomposes) | ||
| Conjugate base | Bicarbonate, Carbonate | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
In chemistry, carbonic acid is a dibasic acid with the chemical formula H2CO3. The pure compound decomposes at temperatures greater than ca. −80 °C.[2]
In biochemistry, the name "carbonic acid" is often applied to aqueous solutions of carbon dioxide, which play an important role in the bicarbonate buffer system, used to maintain acid–base homeostasis.[3]
Chemical equilibria[]
Equilibrium constant values[]
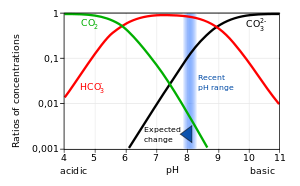
In aqueous solution carbonic acid behaves as a dibasic acid. The Bjerrum plot shows typical equilibrium concentrations, in solution, in seawater, of carbon dioxide and the various species derived from it, as a function of pH.[4] The acidification of natural waters is caused by the increasing concentration of carbon dioxide in the atmosphere, which is believed to be caused by the burning of increasing amounts of coal and hydrocarbons.[5][6]
Expected change refers to predicted effect of continued ocean acidification.[7] It has been estimated that the increase in dissolved carbon dioxide has caused the ocean's average surface pH to decrease by about 0.1 from pre-industrial levels.
The stability constants database contains 136 entries with values for the overall protonation constants, β1 and β2, of the carbonate ion. In the following expressions [H+] represents the concentration, at equilibrium, of the chemical species H+, etc.
The value of log β1 decreases with increasing ionic strength, . At 25 °C:
- :
- (selected data from SC-database)
The value of log β2 also decreases with increasing ionic strength.
- :
At =0 and 25 °C the pK values of the stepwise dissociation constants are
- pK1 = logβ2 - logβ1 = 6.77.
- pK2 = logβ1 = 9.93.
When pH = pK the two chemical species in equilibrium with each other have the same concentration.
Note 1: There are apparently conflicting values in the literature for pKa. Pines et al. cite a value for "pKapp" of 6.35, consistent with the value 6.77, mentioned above.[8] They also give a value for "pKa" of 3.49 and state that
- pKa = pKapp − log KD (eqn. 5)
where KD=[CO2]/[H2CO3]. (eqn. 3) The situation arises from the way that the dissociation constants are named and defined, which is clearly stated in the text of the Pines paper, but not in the abstract.
Note 2: The numbering of dissociation constants is the reverse of the numbering of the numbering of association constants, so pK2 (dissociation)= log β1 (association). The value of the stepwise constant for the equilibrium
is given by
- pK1(dissociation)1 = log β2 − log β1 (association)
In non-biological solutions[]
The hydration equilibrium constant at 25 °C is called Kh, which in the case of carbonic acid is [H2CO3]/[CO2] ≈ 1.7×10−3 in pure water[9] and ≈ 1.2×10−3 in seawater.[10] Hence, the majority of the carbon dioxide is not converted into carbonic acid, remaining as CO2 molecules. In the absence of a catalyst, the equilibrium is reached quite slowly. The rate constants are 0.039 s−1 for the forward reaction and 23 s−1 for the reverse reaction.
In nature, limestone may react with rainwater, forming a solution of calcium bicarbonate; evaporation of such a solution will result re-formation of solid calcium carbonate. These processes occur in the formation of stalactites and stalagmites.
In biological solutions[]
When the enzyme carbonic anhydrase is also present in the solution the following reaction takes precedence.[11]
When the amount of carbon dioxide created by the forward reaction exceeds its solubility, gas is evolved and a third equilibrium
must also be taken into consideration. The equilibrium constant for this reaction is defined by Henry's law. The two reactions can be combined for the equilibrium in solution.
- :
When Henry's law is used to calculate the value of the term in the denominator care is needed with regard to dimensionality.
In physiology, carbon dioxide excreted by the lungs may be called volatile acid or respiratory acid.
Use of the term carbonic acid[]

Strictly speaking the term "carbonic acid" refers to the chemical compound with the formula .
Since pKa1 has a value of ca. 6.8 , at equilibrium carbonic acid will be almost 50% dissociated in the extracellular fluid (cytosol) which has a pH of ca.7.2. Note that dissolved carbon dioxide in extracellular fluid is often called as "carbonic acid" in biochemistry literature, for historical reasons. The reaction in which it is produced
- HCO3− + H+ ⇌ CO2 + H2O
is fast in biological systems. Carbon dioxide can be described as the anhydride of carbonic acid.
Pure carbonic acid[]
Carbonic acid, H2CO3, is stable at ambient temperatures in strictly anhydrous conditions. It decomposes to form carbon dioxide in the presence of any water molecules.[12]
Carbonic acid forms as a by-product of CO2/H2O irradiation, in addition to carbon monoxide and radical species (HCO and CO3).[2] Another route to form carbonic acid is protonation of bicarbonates (HCO3−) with aqueous HCl or HBr. This has to be done at cryogenic conditions to avoid immediate decomposition of H2CO3 to CO2 and H2O.[13] Amorphous H2CO3 forms above 120 K, and crystallization takes place above 200 K to give "β-H2CO3", as determined by infrared spectroscopy. The spectrum of β-H2CO3 agrees very well with the by-product after CO2/H2O irradiation.[2] β-H2CO3 sublimes at 230–260 K largely without decomposition. Matrix-isolation infared spectroscopy allows for the recording of single molecules of H2CO3.[14]
The fact that the carbonic acid may form by irradiating a solid H2O + CO2 mixture or even by proton-implantation of dry ice alone[15] has given rise to suggestions that H2CO3 might be found in outer space or on Mars, where frozen ices of H2O and CO2 are found, as well as cosmic rays.[12] The surprising stability of sublimed H2CO3 up to rather high-temperatures of 260 K even allows for gas-phase H2CO3, e.g., above the pole caps of Mars.[14] Ab initio calculations showed that a single molecule of water catalyzes the decomposition of a gas-phase carbonic acid molecule to carbon dioxide and water. In the absence of water, the dissociation of gaseous carbonic acid is predicted to be very slow, with a half-life in the gas-phase of 180,000 years at 300 K.[12] This only applies if the molecules are few and far apart, because it has also been predicted that gas-phase carbonic acid will catalyze its own decomposition by forming dimers, which then break apart into two molecules each of water and carbon dioxide.[16]
Solid "α-carbonic acid" was claimed to be generated by a cryogenic reaction of potassium bicarbonate and a solution of HCl in methanol.[17][18] This claim was disputed in a PhD thesis submitted in January 2014.[19] Instead, isotope labeling experiments point to the involvement of carbonic acid monomethyl ester (CAME). Furthermore, the sublimed solid was suggested to contain CAME monomers and dimers, not H2CO3 monomers and dimers as previously claimed.[20] Subsequent matrix-isolation infrared spectra confirmed that CAME rather than carbonic acid is found in the gas-phase above "α-carbonic acid".[21] The assignment as CAME is further corroborated by matrix-isolation of the substance prepared in gas-phase by pyrolysis.[22]
Despite its complicated history, carbonic acid may still appear as distinct polymorphs. Carbonic acid forms upon oxidization of CO with OH-radicals.[23] It is not clear whether carbonic acid prepared in this way needs to be considered as γ-H2CO3. The structures of β-H2CO3 and γ-H2CO3 have not been characterized crystallographically.
At high pressure[]
Although molecules of H2CO3 do not constitute a significant portion of the dissolved carbon in aqueous "carbonic acid" under ambient conditions, significant amounts of molecular H2CO3 can exist in aqueous solutions subjected to pressures of multiple gigapascals (tens of thousands of atmospheres), such as can occur in planetary interiors.[24][25]
Carbonic acid should be stabilized under pressures of 0.6–1.6 GPa at 100 K, and 0.75–1.75 GPa at 300 K. These pressures are attained in the cores of large icy satellites such as Ganymede, Callisto, and Titan, where water and carbon dioxide are present. Pure carbonic acid, being denser, would then sink under the ice layers and separate them from the rocky cores of these moons.[26]
References[]
- ^ "Front Matter". Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. pp. P001–P004. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- ^ Jump up to: a b c M. H. Moore; R. K. Khanna (1990). "Infrared and mass spectral studies of proton irradiated H2O + CO2 ice: Evidence for carbonic acid". Spectrochimica Acta Part A. 47 (2): 255–262. Bibcode:1991AcSpA..47..255M. doi:10.1016/0584-8539(91)80097-3.
- ^ Acid-Base Physiology 2.1 – Acid-Base Balance by Kerry Brandis.
- ^ Andersen, C. B. (2002). "Understanding carbonate equilibria by measuring alkalinity in experimental and natural systems". Journal of Geoscience Education. 50 (4): 389–403. Bibcode:2002JGeEd..50..389A. doi:10.5408/1089-9995-50.4.389. S2CID 17094010.
- ^ Caldeira, K.; Wickett, M. E. (2003). "Anthropogenic carbon and ocean pH". Nature. 425 (6956): 365. Bibcode:2001AGUFMOS11C0385C. doi:10.1038/425365a. PMID 14508477. S2CID 4417880.
- ^ Sabine, C. L.; et al. (2004). "The Oceanic Sink for Anthropogenic CO2". Science. 305 (5682): 367–371. Bibcode:2004Sci...305..367S. doi:10.1126/science.1097403. hdl:10261/52596. PMID 15256665. S2CID 5607281. Archived from " the original on 6 July 2008. Retrieved 22 June 2021.
- ^ National Research Council. "Summary". Ocean Acidification: A National Strategy to Meet the Challenges of a Changing Ocean. Washington, DC: The National Academies Press, 2010. 1. Print.
- ^ Pines, Dina; Ditkovich, Julia; Mukra, Tzach; Miller, Yifat; Kiefer, Philip M.; Daschakraborty, Snehasis; Hynes, James T.; Pines, Ehud (2016). "How Acidic Is Carbonic Acid?". J Phys Chem B. 120 (9): 2440–2451. doi:10.1021/acs.jpcb.5b12428. PMC 5747581. PMID 26862781.
- ^ Housecroft and Sharpe, Inorganic Chemistry, 2nd ed, Prentice-Pearson-Hall 2005, p. 368.
- ^ Soli, A. L.; R. H. Byrne (2002). "CO2 system hydration and dehydration kinetics and the equilibrium CO2/H2CO3 ratio in aqueous NaCl solution". Marine Chemistry. 78 (2–3): 65–73. doi:10.1016/S0304-4203(02)00010-5.
- ^ Lindskog S (1997). "Structure and mechanism of carbonic anhydrase". Pharmacology & Therapeutics. 74 (1): 1–20. doi:10.1016/S0163-7258(96)00198-2. PMID 9336012.
- ^ Jump up to: a b c Loerting, Thomas; Tautermann, Christofer; Kroemer, Romano T.; Kohl, Ingrid; Hallbrucker, Andreas; Mayer, Erwin; Liedl, Klaus R.; Loerting, Thomas; Tautermann, Christofer; Kohl, Ingrid; Hallbrucker, Andreas; Erwin, Mayer; Liedl, Klaus R. (2000). "On the Surprising Kinetic Stability of Carbonic Acid (H2CO3)". Angew. Chem. Internat. Edn. 39 (5): 891–894. doi:10.1002/(SICI)1521-3773(20000303)39:5<891::AID-ANIE891>3.0.CO;2-E.
- ^ Hage, Wolfgang; Hallbrucker, Andreas; Mayer, Erwin (1995). "A polymorph of carbonic acid and its possible astrophysical relevance". Journal of the Chemical Society, Faraday Transactions. 91 (17): 2823. Bibcode:1995JCSFT..91.2823H. doi:10.1039/ft9959102823.
- ^ Jump up to: a b Bernard, Jürgen; Huber, Roland G.; Liedl, Klaus R.; Grothe, Hinrich; Loerting, Thomas (14 May 2013). "Matrix Isolation Studies of Carbonic Acid—The Vapor Phase above the β-Polymorph". Journal of the American Chemical Society. 135 (20): 7732–7737. doi:10.1021/ja4020925. PMC 3663070. PMID 23631554.
- ^ Brucato, J; Palumbo, M; Strazzulla, G (January 1997). "Carbonic Acid by Ion Implantation in Water/Carbon Dioxide Ice Mixtures". Icarus. 125 (1): 135–144. doi:10.1006/icar.1996.5561.
- ^ de Marothy, S. A. (2013). "Autocatalytic decomposition of carbonic acid". Int. J. Quantum Chem. 113 (20): 2306–2311. doi:10.1002/qua.24452.
- ^ Hage, W.; Hallbrucker, A.; Mayer, E. (1993). "Carbonic Acid: Synthesis by Protonation of Bicarbonate and FTIR Spectroscopic Characterization Via a New Cryogenic Technique". J. Am. Chem. Soc. 115 (18): 8427–8431. Bibcode:1993JAChS.115.8427H. doi:10.1021/ja00071a061.
- ^ "Press release: International First: Gas-phase Carbonic Acid Isolated". Technische Universität Wien. 11 January 2011. Archived from the original on 9 August 2017. Retrieved 9 August 2017.
- ^ Bernard, Jürgen (January 2014). Solid and Gaseous Carbonic Acid (PDF) (Ph.D. thesis). University of Innsbruck.
- ^ Bernard, Jürgen; Seidl, Markus; Kohl, Ingrid; Liedl, Klaus R.; Mayer, Erwin; Gálvez, Óscar; Grothe, Hinrich; Loerting, Thomas (18 February 2011). "Spectroscopic Observation of Matrix-Isolated Carbonic Acid Trapped from the Gas Phase". Angewandte Chemie International Edition. 50 (8): 1939–1943. doi:10.1002/anie.201004729. PMID 21328675.
- ^ Köck, Eva‐Maria; Bernard, Jürgen; Podewitz, Maren; Dinu, Dennis F.; Huber, Roland G.; Liedl, Klaus R.; Grothe, Hinrich; Bertel, Erminald; Schlögl, Robert; Loerting, Thomas (26 November 2019). "Alpha‐Carbonic Acid Revisited: Carbonic Acid Monomethyl Ester as a Solid and its Conformational Isomerism in the Gas Phase". Chemistry – A European Journal. 26 (1): 285–305. doi:10.1002/chem.201904142. PMC 6972543. PMID 31593601.
- ^ Reisenauer, H. P.; Wagner, J. P.; Schreiner, P. R. (2014). "Gas-Phase Preparation of Carbonic Acid and Its Monomethyl Ester". Angew. Chem. Int. Ed. 53 (44): 11766–11771. doi:10.1002/anie.201406969. PMID 25196920.
- ^ Oba, Yasuhiro; Watanabe, Naoki; Kouchi, Akira; Hama, Tetsuya; Pirronello, Valerio (20 October 2010). "Formation Of Carbonic Acid (H2CO3) By Surface Reactions Of Non-Energetic OH Radicals With CO Molecules At Low Temperatures". The Astrophysical Journal. 722 (2): 1598–1606. Bibcode:2010ApJ...722.1598O. doi:10.1088/0004-637X/722/2/1598.
- ^ Wang, Hongbo; Zeuschner, Janek; Eremets, Mikhail; Troyan, Ivan; Williams, Jonathon (27 January 2016). "Stable solid and aqueous H2CO3 from CO2 and H2O at high pressure and high temperature". Scientific Reports. 6 (1): 19902. Bibcode:2016NatSR...619902W. doi:10.1038/srep19902. PMC 4728613. PMID 26813580.
- ^ Stolte, Nore; Pan, Ding (4 July 2019). "Large presence of carbonic acid in CO2-rich aqueous fluids under Earth's mantle conditions" (PDF). The Journal of Physical Chemistry Letters. 10 (17): 5135–5141. arXiv:1907.01833. doi:10.1021/acs.jpclett.9b01919. PMID 31411889. S2CID 195791860. Retrieved 24 March 2021.
- ^ G. Saleh; A. R. Oganov (2016). "Novel Stable Compounds in the C-H-O Ternary System at High Pressure". Scientific Reports. 6: 32486. Bibcode:2016NatSR...632486S. doi:10.1038/srep32486. PMC 5007508. PMID 27580525.
Further reading[]
 "Climate and Carbonic Acid" in Popular Science Monthly Volume 59, July 1901
"Climate and Carbonic Acid" in Popular Science Monthly Volume 59, July 1901- Welch, M. J.; Lifton, J. F.; Seck, J. A. (1969). "Tracer studies with radioactive oxygen-15. Exchange between carbon dioxide and water". J. Phys. Chem. 73 (335): 3351. doi:10.1021/j100844a033.
- Jolly, W. L. (1991). Modern Inorganic Chemistry (2nd Edn.). New York: McGraw-Hill. ISBN 978-0-07-112651-9.
- Moore, M. H.; Khanna, R. (1991). "Infrared and Mass Spectral Studies of Proton Irradiated H2O+Co2 Ice: Evidence for Carbonic Acid Ice: Evidence for Carbonic Acid". Spectrochimica Acta. 47A (2): 255–262. Bibcode:1991AcSpA..47..255M. doi:10.1016/0584-8539(91)80097-3.
- W. Hage, K. R. Liedl; Liedl, E.; Hallbrucker, A; Mayer, E (1998). "Carbonic Acid in the Gas Phase and Its Astrophysical Relevance". Science. 279 (5355): 1332–1335. Bibcode:1998Sci...279.1332H. doi:10.1126/science.279.5355.1332. PMID 9478889.
- Hage, W.; Hallbrucker, A.; Mayer, E. (1995). "A Polymorph of Carbonic Acid and Its Possible Astrophysical Relevance". J. Chem. Soc. Faraday Trans. 91 (17): 2823–2826. Bibcode:1995JCSFT..91.2823H. doi:10.1039/ft9959102823.
External links[]
- Alkanoic acids
- Carbonates
- Mineral acids




![{\displaystyle \beta _{1}={\frac {[{\text{HCO}}_{3}^{-}]}{[{\text{H}}^{+}][{\text{CO}}_{3}^{2-}]}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/170497229ff9169a1bf46ef141b7f8f0e4156143)


![{\displaystyle \beta _{2}={\frac {[{\text{H}}_{2}{\text{CO}}_{3}]}{[{\text{H}}^{+}]^{2}[{\text{CO}}_{3}^{2-}]}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/b8c36da403b35d01cd677b5d8be799b358f2736a)





![{\displaystyle {\ce {K_3 = {\frac {[H^+][HCO_3^{-}]}{[CO_2(soln)]}}}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/728be989d8b5ded1f0cf378c3ebe5f6532b00c8f)
