Chaconine
| |
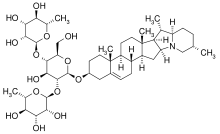
 3D model of chaconine using MolView
| |
| Names | |
|---|---|
| IUPAC name
(3β)-Solanid-5-en-3-yl O-6-deoxy-α-L-mannopyranosyl-(1→2)-O-(6-deoxy-α-L-mannopyranosyl-(1→4))-β-D-glucopyranoside
| |
| Preferred IUPAC name
(2S,2′S,3R,3′R,4R,4′R,5R,5′R,6S,6′S)-2,2′-{[(2R,3S,4S,5R,6R)-4-Hydroxy-2-(hydroxymethyl)-6-{[(2S,4aR,4bS,6aS,6bR,7S,7aR,10S,12aS,13aS,13bS)-4a,6a,7,10-tetramethyl-1,3,4,4a,4b,5,6,6a,6b,7,7a,8,9,10,11,12a,13,13a,13b,14-icosahydro-2H-naphtho[2′,1′:4,5]indeno[1,2-b]indolizin-2-yl]oxy}oxane-3,5-diyl]bis(oxy)}bis(6-methyloxane-3,4,5-triol) | |
| Other names
α-Chaconine, Chaconine
| |
| Identifiers | |
3D model (JSmol)
|
|
| 77396 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.161.828 |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C45H73NO14 | |
| Molar mass | 852.072 g·mol−1 |
| Melting point | 243 °C (469 °F; 516 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
α-Chaconine is a steroidal glycoalkaloid that occurs in plants of the family Solanaceae. It is a natural toxicant produced in green potatoes and gives the potato a bitter taste.[1] Tubers produce this glycoalkaloid in response to stress, providing the plant with insecticidal and fungicidal properties.[1] It belongs to the chemical family of saponins. Since it causes physiological effects on individual organism, chaconine is considered to be defensive allelochemical.[2] Solanine is a related substance that has similar properties.
Symptoms and treatment[]
These are similar to symptoms from ingesting solanine. There are a wide variety of symptoms including: abdominal pain, diarrhea, headache etc.[3]
There is no medicine for detoxification but if it is just after consumption, taking laxative or Gastric lavage could be effective. The symptoms could last several days.
Toxicity[]
Presence of more than 20 mg/100g tuber glycoalkaloids are toxic for humans.[4]
There are death cases cases when potatoes with high glycoalkaloids content.[5] However, it is rare.[6]
Some researches show teratogenic effects on humans but epidemiological investigations produced conflicting research as well.[5] Content of glycoalkaloids most likely differ by cultivar, storage conditions (especially exposure to sunlight), processing techniques.[5]
Difference between Chaconine and Solanine[]
Structural difference[]
Although α-chaconine and α-solanine are both derived from solanidine, the difference appears in 3 groups attached to the terminal oxygen in solanidine. For α-chaconine, these groups are one D-glucose and two L-rhamnose whereas in α-solanine, they are D-galactose, D-glucose, and L-rhamnose.
Difference in Toxicity[]
In an experiment demonstrating the feeding-inhibition effect of solanine and chaconine on snails, chaconine had a greater effect than solanine. However, a mixture of chaconine and solanine had a synergistic effect. The mixture had a significantly higher effect of deterred feeding than using solanine and chaconine on their own.[7]
Ratio of ɑ-chaconine to ɑ-solanine in potato[]
On average, it is between 1.2 and 2.6 to 1, meaning the amount of ɑ-chaconine is greater than ɑ-solanine.[8] However, the average ratio for the peel was 2.0 whereas that for the flesh was nearly 1.5. Also, the ratio was not consistent and depended on cultivar, growth condition, and method of storage.[8][3]
Research on Glycoalkaloids[]
Controlling Amount of Steroidal Glycoalkaloids in Potato[]
In 2014, a research group in Japan, from Institute of Physical and Chemical Research (or RIKEN) found genes for enzymes that are involved in the synthesis of cholesterol, cycloartanol, and related steroidal glycoalkaloids (SGAs), SSR2. Since SGAs are biosynthesized from cholesterol, restricting those enzymes could reduce the amount of the SGAs in potato.[9]
Level of glycoalkaloids[]
The research shows which process of treating potatoes affects the amount of glycoalkaloids (containing both solanine and chaconine more than 90%). The research did four processes which are boiling, baking, frying, and microwaving. As a result, it appears to be frying peel has the largest amount of glycoalkaloids (139–145 mg/100g of product) whereas others contained the average amount of 3 mg/100g product (See the original article for detailed values).[5]
In another research, it showed the amount of SGAs is unaffected by baking, boiling, and frying.[10] This research also shows very high level of SGAs with non-peeleed potato tubers (200 mg kg^-1 FM).
In 2004, there was the research that investigate the change of the amount of α-chaconine and α-solanine over time (90 days). The result showed how the amount of both α-chaconine and α-solanine did not change significantly if it is kept in cold and dark place. The amount actually varied by little but the research concluded that it is due to the cultivar.
The Analytical procedure for this experiment: Take 5 g of sample and homogenize with 15ml of methanol, then filter and make 50 ml of sample extract solution with methanol. Mix 5ml of sample with 12ml of water. Eluate by using Sep-Pak Plus C18 cartridge. Then dry and mix residue with 1ml of methanol and test the solution by HLPC.[11]
Treating poisons in potato[]
Peels and sprouts usually contain high level of SGAs. There are relatively larger amount if tuber is exposed in sunlight. If tubers are not mature enough, those might contain high level of chaconine and solanine. Thus, sprouts on potato and peels should be removed and if there is the green part inside potato, it should be removed as well. It should be preserved in dark and cold place, but it does not have to be in the fridge. It is likely to germinate or degrade when the surrounding is above 20℃. As it is mentioned in earlier chapter, heating might not be very effective towards SGAs, therefore, those contain high level of SGAs should be carefully removed.[12][1] Also, if potato is kept in fridge, it increases the amount of sugar. And when cooking (such as frying, baking), the toxic compound called acrylamide formed when sugar and amino acids react.
When cooking potato, if it is fried at 210℃ for 10 minutes, the amount of solanine and chaconine decreased to 60% of it original amount. If it is fried at 170℃ for 5 minutes, there was no significant change in the amount of solanine and chaconine. But if it is fried for 15 minutes at the same temperature, solanine decreased 76.1% and chaconine decomposed 81.5%. Thus, the break down of solanine and chaconine are considered to start around 170℃.[13] In one research, the solution containing α-solanine and α-chaconine is put into boiled water for 150 minutes, there was no significant decrease in the amount of solanine and chaconine. Therefore, it can be considered that boiling potato is not effective to reduce the amount of solanine and chaconine.[13]
Also, since glycoalkaloids are soluble in water, it is one of ways to put potato into water for a while so that SGAs dissolve into water.[14]
Attempt to make toxin-free potato[]
In 2015, the research was launched that tries to make potato with no glycoalkaloids by genome editing. There is a cost for checking the amount of glycoalkaloids since it heavily affects human health. Thus, if there is such potato with no toxin, it is not only beneficial for humans in terms of health but also, there would be much less need for money spent on examination of glycoalkaloids for potato. In this research, it also asks about why glycoalkaloids exists since there seems to be not much benefits gained from it. The researcher for this website says how many of those researches that mentions about the effect of glycoalkaloids are not trustworthy except one (by S. L. Sinden in 1986[15]).[16]
See also[]
References[]
- ^ a b c Kuiper-Goodman, T.; Nawrot, P.S. "Toxin profile: Solanine and Chaconine IPCS, INCHEM". Retrieved 2021-03-15.
- ^ Saponins used in traditional and modern medicine. Boston, MA: Springer. 1996. pp. 277–295. ISBN 978-1-4899-1369-2.
- ^ a b McKenzie, Marian; Corrigan, Virginia (1 January 2016). "Chapter 12 - Potato Flavor". Advances in Potato Chemistry and Technology (Second Edition): 339–368. doi:10.1016/B978-0-12-800002-1.00012-1.
- ^ "Potato Flavor and Texture". doi:10.1016/B978-044451018-1/50066-X.
- ^ a b c d "a-Chaconine and a-Solanine Content of Potato Products and Their Stability during Several Modes of Cooking". Journal of Agricultural and Food Chemistry. doi:10.1021/jf00106a033.
- ^ "Potato plant poisoning - green tubers and sprouts".
- ^ Smith, David B.; Roddick, James G.; Jones, J.Leighton (May 2001). "Synergism between the potato glycoalkaloids α-chaconine and α-solanine in inhibition of snail feeding". Phytochemistry. 57 (2): 229–234. doi:10.1016/S0031-9422(01)00034-6.
- ^ a b Friedman, Mendel; Levin, Carol E. (2009). "Analysis and Biological Activities of Potato Glycoalkaloids, Calystegine Alkaloids, Phenolic Compounds, and Anthocyanins". Advances in Potato Chemistry and Technology: 127–161. doi:10.1016/B978-0-12-374349-7.00006-4.
- ^ Sawai, S.; Ohyama, K.; Yasumoto, S.; Seki, H.; Sakuma, T.; Yamamoto, T.; Takebayashi, Y.; Kojima, M.; Sakakibara, H.; Aoki, T.; Muranaka, T.; Saito, K.; Umemoto, N. (1 September 2014). "Sterol Side Chain Reductase 2 Is a Key Enzyme in the Biosynthesis of Cholesterol, the Common Precursor of Toxic Steroidal Glycoalkaloids in Potato". The Plant Cell. 26 (9): 3763��3774. doi:10.1105/tpc.114.130096.
- ^ "Balance between nutrients and anti-nutrients in nine Italian potato cultivars". Food Chemistry. Retrieved 2021-03-15.
- ^ "Contents and its Change during Storage of a-Solanine and a-Chaconine in Potatoes". doi:10.3358/shokueishi.45.277. Retrieved 2021-03-15.
- ^ "Review of Toxicological Literature" (PDF). Retrieved 2021-03-15.
- ^ a b "Food hygiene and safety science". Food Hygiene and Safety Science: 67–73. 1990. doi:10.3358/shokueishi.31.67. Retrieved 2021-03-15.
- ^ "Changes in the Levels of Glycoalkaloids and Nitrates After the Dehydration of Cooked Potatoes". American Journal of Potato Research. doi:10.1007/s12230-012-9273-0. Retrieved 2021-03-15.
- ^ "Leptine Glycoalkaloids and Resistance to the Colorado Potato Beetle (Coleoptera: Chrysomelidae) in Solanum chacoense". Environmental Entomology. doi:10.1093/ee/15.5.1057. Retrieved 2021-03-15.
- ^ Umemoto, Naoyuki. "Is It Possible to Breed Toxin-Free Potato?: Identification and Application of Glycoalkaloid Biosynthetic Genes".
External links[]
 Media related to Chaconine at Wikimedia Commons
Media related to Chaconine at Wikimedia Commons
- Steroidal alkaloids
- Alkaloid glycosides
- Saponins
- Plant toxins
- Steroidal alkaloids found in Solanaceae