Saponin
Saponins (Latin "sapon", soap + “-in", one of), also referred to selectively as triterpene glycosides, are bitter-tasting usually toxic plant-derived organic chemicals that have a foamy quality when agitated in water. They are widely distributed but found particularly in soapwort (genus Saponaria), a flowering plant, and the soapbark tree (Quillaja saponaria). They are used in soaps, medicinals, fire extinguishers, speciously as dietary supplements, for synthesis of steroids, and in carbonated beverages (the head on a mug of root beer). Structurally, they are glycosides, sugars attached to another organic molecule, usually a steroid or triterpene, a steroid building block. Saponins are both water and fat soluble, which gives them their useful soap properties. Some examples of these chemicals are glycyrrhizin, licorice flavoring; and quillaia(alt. quillaja), a bark extract used in beverages.[1][2]
Uses[]
The saponins are a subclass of terpenoids, the largest class of plant extracts. The amphipathic nature of saponins gives them activity as surfactants with potential ability to interact with cell membrane components, such as cholesterol and phospholipids, possibly making saponins useful for development of cosmetics and drugs.[3] Saponins have also been used as adjuvants in development of vaccines,[4] such as Quil A, an extract from the bark of Quillaja saponaria.[3][5] This makes them of interest for possible use in subunit vaccines and vaccines directed against intracellular pathogens.[4] In their use as adjuvants for manufacturing vaccines, toxicity associated with sterol complexation remains a concern.[6]
While saponins are promoted commercially as dietary supplements and are used in traditional medicine, there is no high-quality clinical evidence that they have any beneficial effect on human health.[5] Quillaja is toxic when consumed in large amounts, involving possible liver damage, gastric pain, diarrhea, or other adverse effects.[5]
Saponins are used for their effects on ammonia emissions in animal feeding.[7] In the United States, researchers are exploring the use of saponins derived from plants to control invasive worm species, including the jumping worm.[8][9]
Decoction[]
The principal historical use of these plants was in the making of soap. Saponaria officinalis is most suited for this procedure, but other related species also work. The greatest concentration of saponin will be when the plant is flowering, and the most saponin is found in the woody parts of the plant like thick stems and roots, but the leaves also have some.
Sources[]
Saponins have historically been plant-derived, but they have also been isolated from marine organisms such as sea cucumber.[1][10] They[1][11] derive their name from the soapwort plant (genus Saponaria, family Caryophyllaceae), the root of which was used historically as a soap.[2] Saponins are also found in the botanical family Sapindaceae, including its defining genus Sapindus (soapberry or soapnut) and the horse chestnut, and in the closely related families Aceraceae (maples) and Hippocastanaceae. It is also found heavily in Gynostemma pentaphyllum (Cucurbitaceae) in a form called gypenosides, and ginseng or red ginseng (Panax, Araliaceae) in a form called ginsenosides. Saponins are also found in the unripe fruit of Manilkara zapota (also known as sapodillas), resulting in highly astringent properties. Nerium oleander (Apocynaceae), also known as White Oleander, is a source of the potent cardiac toxin oleandrin. Within these families, this class of chemical compounds is found in various parts of the plant: leaves, stems, roots, bulbs, blossom and fruit.[12] Commercial formulations of plant-derived saponins, e.g., from the soap bark tree, Quillaja saponaria, and those from other sources are available via controlled manufacturing processes, which make them of use as chemical and biomedical reagents.[13]
Role in plant ecology and impact on animal foraging[]
In plants, saponins may serve as anti-feedants,[2][14] and to protect the plant against microbes and fungi.[citation needed] Some plant saponins (e.g. from oat and spinach) may enhance nutrient absorption and aid in animal digestion. However, saponins are often bitter to taste, and so can reduce plant palatability (e.g., in livestock feeds), or even imbue them with life-threatening animal toxicity.[14] Some saponins are toxic to cold-blooded organisms and insects at particular concentrations.[14] Further research is needed to define the roles of these natural products in their host organisms, which have been described as "poorly understood" to date.[14]
Ethnobotany[]
Most saponins, which readily dissolve in water, are poisonous to fish.[15] Therefore, in ethnobotany, they are primarily known for their use by indigenous people in obtaining aquatic food sources. Since prehistoric times, cultures throughout the world have used fish-killing plants, mostly those containing saponins, for fishing.[16][17]
Although prohibited by law, fish-poison plants are still widely used by indigenous tribes in Guyana.[18]
On the Indian subcontinent, the Gondi people are known for their use of poison-plant extracts in fishing.[19]
Many of California's Native American tribes traditionally used soaproot, (genus Chlorogalum) and/or the root of various yucca species, which contain saponin, as a fish poison. They would pulverize the roots, mixing in water to create a foam, and then add the suds to a stream. This would kill, or incapacitate, the fish, which could be gathered easily from the surface of the water. Among the tribes using this technique were the Lassik, the Luiseño, and the Mattole.[20]
Chemical structure[]
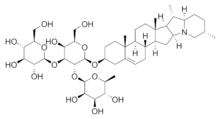
The vast heterogeneity of structures underlying this class of compounds makes generalizations fuzzy; they're a subclass of terpenoids, derivatives of a smelly oily cyclic hydrocarbon, terpene (the alternate steroid base is a terpene missing a few carbon atoms). The derivatives are formed by substituting (usually oxygen-containing) other groups for some of the hydrogens. In the case of most saponins, one of these substituents is a sugar, so the compound is a glycoside of the base molecule. Specifically, the base or fat-soluble portion of a saponin can be a triterpene, a steroid (spirostanol or furostanol) or a steroidal alkyloid (in which nitrogen atoms replace one or more carbon atoms). Another possible base structure is an open (acyclic) side chain instead of the ring structure in the steroid base. One or two (rarely three) water-soluble monosaccharide (simple sugar) chains may bind to the base via hydroxyl (OH) groups, and sometimes there are other substituents such as hydroxyl, hydroxymethyl, carboxyl and acyl groups. The chains may be from 1-11 molecules long, but are usually 2–5, and may include branched chains. The most common such sugars are dietary simple sugars like glucose and galactose, though a wide variety of sugars occur naturally. Other kinds of molecules like organic acids and esters may also attach to the base via carboxyl (COOH) groups. In particular among these are the sugar acids, such as glucuronic acid and galacturonic acid, which are oxidated forms of the sugar.[1]
See also[]
- Phytochemical
- Cardenolide
- Cardiac glycoside
References[]
- ^ Jump up to: a b c d Hostettmann, K.; A. Marston (1995). Saponins. Cambridge: Cambridge University Press. p. 3ff. ISBN 978-0-521-32970-5. OCLC 29670810.
- ^ Jump up to: a b c "Saponins". Cornell University. 14 August 2008. Retrieved 23 February 2009.
- ^ Jump up to: a b Lorent, Joseph H.; Quetin-Leclercq, Joëlle; Mingeot-Leclercq, Marie-Paule (28 November 2014). "The amphiphilic nature of saponins and their effects on artificial and biological membranes and potential consequences for red blood and cancer cells". Organic and Biomolecular Chemistry. Royal Society of Chemistry. 12 (44): 8803–8822. doi:10.1039/c4ob01652a. ISSN 1477-0520. PMID 25295776. S2CID 205925983.
- ^ Jump up to: a b Sun, Hong-Xiang; Xie, Yong; Ye, Yi-Ping (2009). "Advances in saponin-based adjuvants". Vaccine. 27 (12): 1787–1796. doi:10.1016/j.vaccine.2009.01.091. ISSN 0264-410X. PMID 19208455.
- ^ Jump up to: a b c "Quillaja". Drugs.com. 2018. Retrieved 26 December 2018.
- ^ Skene, Caroline D.; Philip Sutton (1 September 2006). "Saponin-adjuvanted particulate vaccines for clinical use". Methods. 40 (1): 53–9. doi:10.1016/j.ymeth.2006.05.019. PMID 16997713.
- ^ Zentner, Eduard (July 2011). "Effects of phytogenic feed additives containing quillaja saponaria on ammonia in fattening pigs" (PDF). Retrieved 27 November 2012.
- ^ Roach, Margaret (22 July 2020). "As Summer Takes Hold, So Do the Jumping Worms". The New York Times. ISSN 0362-4331. Retrieved 30 July 2020.
- ^ "Invasive 'Jumping' Worms Are Now Tearing Through Midwestern Forests". Audubon. 2 January 2020. Retrieved 30 July 2020.
- ^ Riguera, Ricardo (August 1997). "Isolating bioactive compounds from marine organisms". Journal of Marine Biotechnology. 5 (4): 187–193.[dead link]
- ^ Liener, Irvin E (1980). Toxic constituents of plant foodstuffs. The Proceedings of the Nutrition Society. 29. New York City: Academic Press. pp. 56–7. doi:10.1079/pns19700010. ISBN 978-0-12-449960-7. OCLC 5447168. PMID 5529217.[verification needed]
- ^ http://sun.ars-grin.gov:8080/npgspub/xsql/duke/plantdisp.xsql?taxon=691
- ^ "Saponin from quillaja bark". Sigma-Aldrich. Retrieved 23 February 2009.
- ^ Jump up to: a b c d Foerster, Hartmut (22 May 2006). "MetaCyc Pathway: saponin biosynthesis I". Retrieved 23 February 2009.
- ^ Howes, F. N. (1930), "Fish-poison plants", Bulletin of Miscellaneous Information (Royal Gardens, Kew), 1930 (4): 129–153, doi:10.2307/4107559, JSTOR 4107559
- ^ Jonathan G. Cannon, Robert A. Burton, Steven G. Wood, and Noel L. Owen (2004), "Naturally Occurring Fish Poisons from Plants", J. Chem. Educ., 81 (10): 1457, Bibcode:2004JChEd..81.1457C, doi:10.1021/ed081p1457CS1 maint: uses authors parameter (link)
- ^ C. E. Bradley (1956), "Arrow and fish poison of the American southwest", Division of Biology, California Institute of Technology, 10 (4), pp. 362–366, doi:10.1007/BF02859766, S2CID 35055877
- ^ Tinde Van Andel (2000), "The diverse uses of fish-poison plants in Northwest Guyana", Economic Botany, 54 (4): 500–512, doi:10.1007/BF02866548, hdl:1874/23514, S2CID 24945604
- ^ Murthy E N, Pattanaik, Chiranjibi, Reddy, C Sudhakar, Raju, V S (March 2010), "Piscicidal plants used by Gond tribe of Kawal wildlife sanctuary, Andhra Pradesh, India", Indian Journal of Natural Products and Resources, 1 (1): 97–101CS1 maint: multiple names: authors list (link)
- ^ Campbell, Paul (1999). Survival skills of native California. Gibbs Smith. p. 433. ISBN 978-0-87905-921-7.
| Wikimedia Commons has media related to Saponins. |
- Saponins
- Saponaceous plants
