Terpenoid
The terpenoids, also known as isoprenoids, are a large and diverse class of naturally occurring organic chemicals derived from the 5-carbon compound isoprene, and the isoprene polymers called terpenes. While sometimes used interchangeably with "terpenes", terpenoids contain additional functional groups, usually containing oxygen.[1] Terpenoids are the largest class of plant secondary metabolites, representing about 60% of known natural products.[2] Many terpenoids have substantial pharmacological bioactivity and are therefore of interest to medicinal chemists.[3]
Plant terpenoids are used for their aromatic qualities and play a role in traditional herbal remedies. Terpenoids contribute to the scent of eucalyptus, the flavors of cinnamon, cloves, and ginger, the yellow color in sunflowers, and the red color in tomatoes.[4] Well-known terpenoids include citral, menthol, camphor, salvinorin A in the plant Salvia divinorum, the cannabinoids found in cannabis, ginkgolide and bilobalide found in Ginkgo biloba, and the curcuminoids found in turmeric and mustard seed. The provitamin beta carotene is a terpene derivative called a carotenoid.
The steroids and sterols in animals are biologically produced from terpenoid precursors. Sometimes terpenoids are added to proteins, e.g., to enhance their attachment to the cell membrane; this is known as isoprenylation.
Structure and classification[]
Terpenoids are modified terpenes,[5] wherein methyl groups have been moved or removed, or oxygen atoms added. Some authors use the term "terpene" more broadly, to include the terpenoids. Just like terpenes, the terpenoids can be classified according to the number of isoprene units that comprise the parent terpene:
| Terpenoids | Analogue terpenes | Number of isoprene units | Number of carbon atoms | General formula | Examples[6] |
|---|---|---|---|---|---|
| Hemiterpenoids | Isoprene | 1 | 5 | C5H8 | DMAPP, isopentenyl pyrophosphate, isoprenol, isovaleramide, isovaleric acid, HMBPP, prenol |
| Monoterpenoids | Monoterpenes | 2 | 10 | C10H16 | Bornyl acetate, camphor, carvone, citral, citronellal, citronellol, geraniol, eucalyptol, hinokitiol, iridoids, linalool, menthol, thymol |
| Sesquiterpenoids | Sesquiterpenes | 3 | 15 | C15H24 | Farnesol, geosmin, humulone |
| Diterpenoids | Diterpenes | 4 | 20 | C20H32 | Abietic acid, ginkgolides, paclitaxel, retinol, salvinorin A, sclareol, steviol |
| Sesterterpenoids | Sesterterpenes | 5 | 25 | C25H40 | Andrastin A, manoalide |
| Triterpenoids | Triterpenes | 6 | 30 | C30H48 | Amyrin, betulinic acid, limonoids, oleanolic acid, sterols, squalene, ursolic acid |
| Tetraterpenoids | Tetraterpenes | 8 | 40 | C40H64 | Carotenoids |
| Polyterpenoid | Polyterpenes | >8 | >40 | (C5H8)n | Gutta-percha, natural rubber |
Terpenoids can also be classified according to the type and number of cyclic structures they contain: linear, acyclic, monocyclic, bicyclic, tricyclic, tetracyclic, pentacyclic, or macrocyclic.[6] The Salkowski test can be used to identify the presence of terpenoids.[7]
- Selected terpenoids
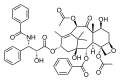
Paclitaxel is a diterpenoid anticancer drug.

Terpineols are monoterpenoids.
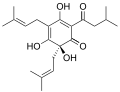
Humulones are classified as sesquiterpenoids.

Retinol is a diterpenoid.

Hinokitiol is a monoterpenoid, a tropolone derivative.

Geosmin is a sesquiterpenoid.
Biosynthesis[]
Terpenoids, at least those containing an alcohol functional group, often arise by hydrolysis of carbocationic intermediates produced from geranyl pyrophosphate. Analogously hydrolysis of intermediates from farnesyl pyrophosphate gives sesquiterpenoids, and hydrolysis of intermediates from geranylgeranyl pyrophosphate gives diterpenoids, etc.[8]
See also[]
- List of antioxidants in food
- List of phytochemicals in food
- Nutrition
- Phytochemistry
- Secondary metabolites
References[]
- ^ Chemistry, International Union of Pure and Applied. IUPAC Compendium of Chemical Terminology. IUPAC. doi:10.1351/goldbook.T06279.
- ^ Firn R (2010). Nature's Chemicals. Oxford: Biology.
- ^ Ashour, Mohamed; Wink, Michael; Gershenzon, Jonathan (2010). "Biochemistry of Terpenoids: Monoterpenes, Sesquiterpenes and Diterpenes". Biochemistry of Plant Secondary Metabolism. pp. 258–303. doi:10.1002/9781444320503.ch5. ISBN 9781444320503.
- ^ Specter M (September 28, 2009). "A Life of Its Own". The New Yorker.
- ^ Houghton, Isaac. "The Physiology of Cannabis Terpenes and Terpenoids – A Brief Overview". Elliot Barker. Retrieved 3 May 2016.
- ^ a b Ludwiczuk, A.; Skalicka-Woźniak, K.; Georgiev, M.I. (2017). "Terpenoids". Pharmacognosy: 233–266. doi:10.1016/B978-0-12-802104-0.00011-1. ISBN 9780128021040.
- ^ Ayoola GA (2008). "Phytochemical Screening and Antioxidant Activities of Some Selected Medicinal Plants Used for Malaria Therapy in Southwestern Nigeria". Tropical Journal of Pharmaceutical Research. 7 (3): 1019–1024. doi:10.4314/tjpr.v7i3.14686.
- ^ Davis, Edward M.; Croteau, Rodney (2000). "Cyclization Enzymes in the Biosynthesis of Monoterpenes, Sesquiterpenes, and Diterpenes". Topics in Current Chemistry. 209: 53–95. doi:10.1007/3-540-48146-X_2. ISBN 978-3-540-66573-1.CS1 maint: uses authors parameter (link)
External links[]
- Terpenes and terpenoids
- Plant communication





