Thujaplicin
| Identifiers | |
|---|---|
| |
3D model (JSmol)
|
|
| ChEBI |
|
| ChEMBL |
|
| EC Number |
|
PubChem CID
|
|
| UNII |
|
CompTox Dashboard (EPA)
|
|
| Properties | |
| C10H12O2 | |
| Molar mass | 164.204 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Thujaplicins (isopropyl cycloheptatrienolones) are a series of tropolone-related chemical substances that have been isolated from the hardwoods of the trees of Cupressaceae family.[1] These compounds are known for their antibacterial, antifungal, and antioxidant properties.[2][3] They were the first natural tropolones to be made synthetically.[4]
History[]

Thujaplicins were discovered in the mid-1930s and purified from the heartwood of Thuja plicata Donn ex D. Don, commonly called as Western red cedar tree.[5] These compounds were also identified in the constituents of Chamaecyparis obtusa, another species from the Cupressaceae family. C. obtusa is native to East Asian countries including Japan and Taiwan, and is also known as Taiwan hinoki, from which the β-thujaplicin was first isolated in 1936 and received its name, hinokitiol. Thujaplicins were the first natural tropolones to be made synthetically, by Ralph Raphael and colleagues, and the β-thujaplicin was the first non-benzenoid aromatic compound identified, by Tetsuo Nozoe and colleagues.[4][5] The resistance of the heartwood of the tree to decay was the main reason prompting to investigate its content and identify the compounds responsible for antimicrobial properties.[4] β-thujaplicin gained more scientific interest beginning in the 2000s.[6] Later, iron-binding activity of β-thujaplicin was discovered and the molecule has been ironically nicknamed as “Iron Man molecule”,[7] because the first name of Tetsuo Nozoe can be translated into English as “Iron Man”.[6]
Occurrence and isolation[]
Tjujaplicins are found in the hardwoods of the trees belonging to the Cupressaceae family, including Chamaecyparis obtusa (Hinoki cypress), Thuja plicata (Western red cedar), Thujopsis dolabrata var. hondai (Hinoki asunaro), Juniperus cedrus (Canary Islands juniper), Cedrus atlantica (Atlas cedar), Cupressus lusitanica (Mexican white cedar), Chamaecyparis lawsoniana (Port Orford cedar), Chamaecyparis taiwanensis (Taiwan cypress), Chamaecyparis thyoides (Atlantic white cedar), Cupressus arizonica (Arizona cypress), Cupressus macnabiana (MacNab cypress), Cupressus macrocarpa (Monterey cypress), Juniperus chinensis (Chinese juniper), Juniperus communis (Common juniper), Juniperus californica (California juniper), Juniperus occidentalis (Western juniper), Juniperus oxycedrus (Cade), Juniperus sabina (Savin juniper), Calocedrus decurrens (California incense-cedar), Calocedrus formosana (Taiwan incense-cedar), Platycladus orientalis (Chinese thuja), Thuja occidentalis (Northern white-cedar), Thuja standishii (Japanese thuja), Tetraclinis articulata (Sandarac).[8][9][10][11]
Thujaplicins can be produced in plant cell suspension cultures,[12][13] or can be extracted from wood using solvents and ultrasonication.[14]
Biosynthesis[]
Thujaplicins can be synthesized by cycloaddition of isopropylcyclopentadiene and dichloroketene, 1,3-dipolar cycloaddition of 5-isopropyl-1-methyl-3-oxidopyridinium, ring expansion of 2-isopropylcyclohexanone, regiocontrolled hydroxylation of oxyallyl (4+3) cycloadducts, from (R)-(+)-limonene regioselectively by several steps, and from troponeirontricarbonyl complex by few steps.[15][16] The synthesis pathway of β-thujaplicin from troponeirontricarbonyl complex is found below:
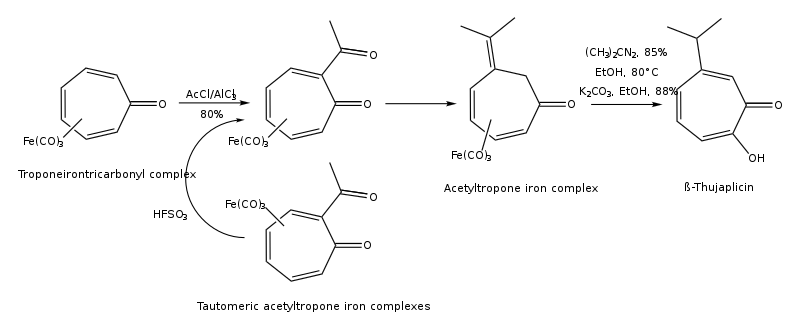
The synthesis pathway of β-thujaplicin by electro-reductive alkylation of substituted cycloheptatrienes is shown below:
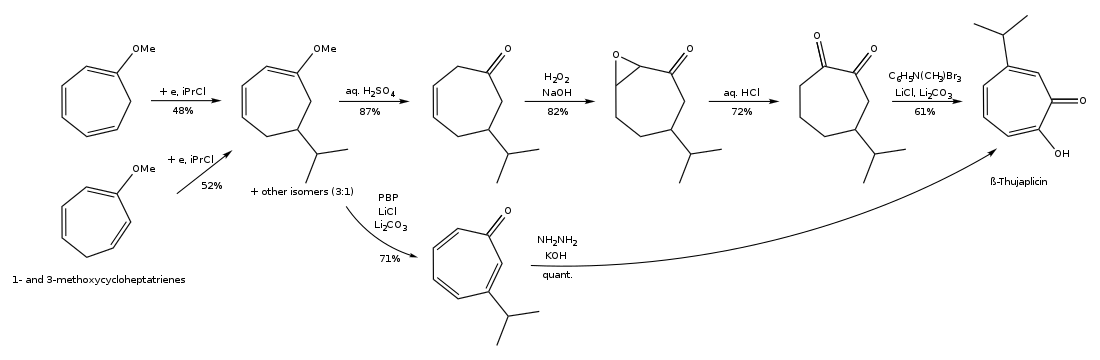
The synthesis pathway of β-thujaplicin through ring expansion of 2-isopropylcyclohexanone is shown below:
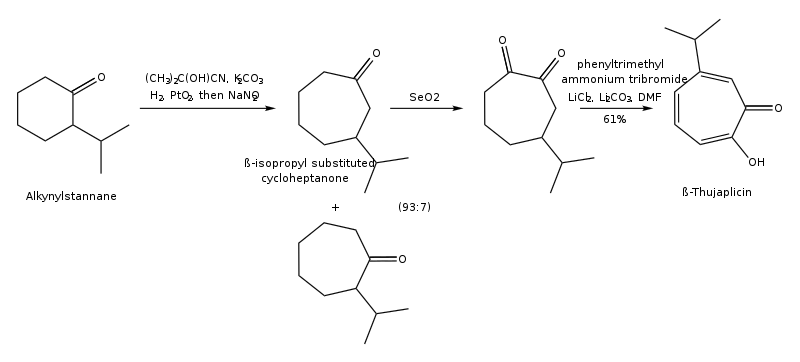
The synthesis pathway of β-thujaplicin through oxyallyl cation [4+3] cyclization (Noyori's synthesis) is shown below:

Chemistry[]
Thujaplicins belong to tropolones containing an unsaturated seven-membered carbon ring. Thujaplicins are monoterpenoids that are cyclohepta-2,4,6-trien-1-one substituted by a hydroxy group at position 2 and an isopropyl group at positions 3, 4 or 5.[17] These compounds are enols and cyclic ketones. They derive from a hydride of a cyclohepta-1,3,5-triene. Thujaplicins are soluble in organic solvents and aqueous buffers. Hinokitiol is soluble in ethanol, dimethyl sulfoxide, dimethylformamide with a solubility of 20, 30 and 12.5 mg/ml, respectively.[18] β-thujaplicin provides acetone on vigorous oxidation and gives the saturated monocyclic diol upon catalytic hydrogenation.[19] It is stable to alkali and acids, forming salts or remaining unchanged, but does not convert to catechol derivatives. The complexes made of iron and tropolones display high thermodynamic stability and has shown to have a stronger binding constant than the trnasferrin-iron complex.[20]
There are three naturally occurring monocyclic tropolones described: α-thujaplicin, β-thujaplicin (hinokitiol), and γ-thujaplicin.[4] And the most common isomer occurring in the nature is β-thujaplicin.[21]
| Compound | Chemical structure | 3D model of the molecule | IUPAC name |
|---|---|---|---|
| α-thujaplicin[22] |  |
 |
2-hydroxy-3-propan-2-ylcyclohepta-2,4,6-trien-1-one |
| β-thujaplicin (hinokitiol)[23] |  |
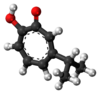 |
2-hydroxy-6-propan-2-ylcyclohepta-2,4,6-trien-1-one |
| γ-thujaplicin[24] | 2-hydroxy-5-propan-2-ylcyclohepta-2,4,6-trien-1-one |
Biological properties[]
Antibacterial and antifungal activity[]
This section is empty. You can help by . (April 2021) |
Antiviral activity[]
This section is empty. You can help by . (April 2021) |
Anti-inflammatory activity[]
This section is empty. You can help by . (April 2021) |
Insecticidal and pesticidal activity[]
Thujaplicins are shown to act against Reticulitermes speratus (Japanese termites), Coptotermes formosanus (super termites), Dermatophagoides farinae (dust mites), Tyrophagus putrescentiae (mould mites), Callosobruchus chinensis (adzuki bean weevil), Lasioderma serricorne (cigarette beetle).[9][25][11]
Hinokitiol has also shown some larvicidal activities against Aedes aegypti (yellow fever mosquito) and Culex pipiens (common house mosquito), and anti-plasmodial activities against Plasmodium falciparum and Plasmodium berghei.[11]
Antioxidant activity[]
This section is empty. You can help by . (April 2021) |
Chelating and ionophore activity[]
Thujaplicins, as other tropolones, demonstrate chelating activity, acting as an ionophore by binding different metal ions.[26]
Anti-browning activity[]
Tropolone and thujaplicins exhibit potent suppressive activity on enzymatic browning due to inhibition of polyphenol oxidase and tyrosinase. This have been shown in experiments on different vegetables, fruits, mushrooms, plants and other agricultural products.[11] Prevention of darkening has also been elicited on seafood products.[27]
Applications[]
Skin care and cosmetics[]
Owing to their antibacterial activities against various microbes colonizing and affecting the skin, thujaplicins are used in skin care and hair growth products,[28] and are especially popular in Eastern Asia.[citation needed]
Oral care[]
Hinokitiol is used in various oral care products, including toothpastes and oral sprays.[28][29]
Veterinary medicine[]
Due to its antifungal activity against Malassezia pachydermatis, it is used in eardrop formulations for external otitis in dogs.[30][31]
Agriculture[]
Considering their antifungal activity against many plant-pathogenic fungi, and pesticidal and insecticidal properties, the role of thujaplicins in agriculture is evolving, including their use in the management of different plant diseases and for controlling the postharvest decay.[9][32]
Food additive[]
Thujaplicins are used as food additives in Japan.[33] Due to its suppressive activity on food browning and the inhibitory activity against bacteria and fungi causing food spoilage (such as Clostridium perfringens, Alternaria alternata, Aspergillus niger, Botrytis cinerea, Fusobacterium species, Monilinia fructicola and Rhizopus stolonifer), hinokitiol is also used in food packaging as a shelf-life extending agent.[34][35][36]
References[]
- ^ ERDTMAN, HOLGER; GRIPENBERG, JARL (May 1948). "Antibiotic Substances from the Heart Wood of Thuja plicata Don". Nature. 161 (4097): 719. doi:10.1038/161719a0. PMID 18860272. S2CID 4074514.
- ^ Chedgy, Russell J.; Lim, Young Woon; Breuil, Colette (May 2009). "Effects of leaching on fungal growth and decay of western redcedar". Canadian Journal of Microbiology. 55 (5): 578–586. doi:10.1139/W08-161. PMID 19483786.
- ^ Chedgy, R. (2010). Secondary Metabolites of Western Red Cedar (Thuja plicata). Lambert Academic Publishing. ISBN 978-3-8383-4661-8.
- ^ Jump up to: a b c d Cook, J. W.; Raphael, R. A.; Scott, A. I. (1951). "149. Tropolones. Part II. The synthesis of α-, β-, and γ-thujaplicins". J. Chem. Soc. 0: 695–698. doi:10.1039/JR9510000695.
- ^ Jump up to: a b Nakanishi, Koji (June 2013). "Tetsuo Nozoe's "Autograph Books by Chemists 1953-1994": An Essay". The Chemical Record. 13 (3): 343–352. doi:10.1002/tcr.201300007. PMID 23737463.
- ^ Jump up to: a b "Hinokitiol". American Chemical Society.
- ^ Service, Robert (11 May 2017). "Iron Man molecule restores balance to cells". Science. doi:10.1126/science.aal1178.
- ^ Okabe, T; Saito, K (1994). "Antibacterial and preservative effects of natural Hinokitiol (beta-Thujaplicin) extracted from wood". Acta Agriculturae Zhejiangensis. 6 (4): 257–266.
- ^ Jump up to: a b c Morita, Yasuhiro; Matsumura, Eiko; Okabe, Toshihiro; Fukui, Toru; Shibata, Mitsunobu; Sugiura, Masaaki; Ohe, Tatsuhiko; Tsujibo, Hiroshi; Ishida, Nakao; Inamori, Yoshihiko (2004). "Biological Activity of α-Thujaplicin, the Isomer of Hinokitiol". Biological & Pharmaceutical Bulletin. 27 (6): 899–902. doi:10.1248/bpb.27.899.
- ^ Rebia, Rina Afiani; binti Sadon, Nurul Shaheera; Tanaka, Toshihisa (22 November 2019). "Natural Antibacterial Reagents (Centella, Propolis, and Hinokitiol) Loaded into Poly[(R)-3-hydroxybutyrate-co-(R)-3-hydroxyhexanoate] Composite Nanofibers for Biomedical Applications". Nanomaterials. 9 (12): 1665. doi:10.3390/nano9121665. PMC 6956080. PMID 31766678.
- ^ Jump up to: a b c d Saniewski, Marian; Horbowicz, Marcin; Kanlayanarat, Sirichai (10 September 2014). "The Biological Activities of Troponoids and Their Use in Agriculture A Review". Journal of Horticultural Research. 22 (1): 5–19. doi:10.2478/johr-2014-0001.
- ^ Zhao, J.; Fujita, K.; Yamada, J.; Sakai, K. (1 April 2001). "Improved β-thujaplicin production in Cupressus lusitanica suspension cultures by fungal elicitor and methyl jasmonate". Applied Microbiology and Biotechnology. 55 (3): 301–305. doi:10.1007/s002530000555. PMID 11341310. S2CID 25767209.
- ^ Yamada, J.; Fujita, K.; Sakai, K. (April 2003). "Effect of major inorganic nutrients on β-thujaplicin production in a suspension culture of Cupressus lusitanica cells". Journal of Wood Science. 49 (2): 172–175. doi:10.1007/s100860300027. S2CID 8355694.
- ^ Chedgy, Russell J.; Daniels, C.R.; Kadla, John; Breuil, Colette (1 March 2007). "Screening fungi tolerant to Western red cedar (Thuja plicata Donn) extractives. Part 1. Mild extraction by ultrasonication and quantification of extractives by reverse-phase HPLC". Holzforschung. 61 (2): 190–194. doi:10.1515/HF.2007.033. S2CID 95994935.
- ^ Soung, Min-Gyu; Matsui, Masanao; Kitahara, Takeshi (September 2000). "Regioselective Synthesis of β- and γ-Thujaplicins". Tetrahedron. 56 (39): 7741–7745. doi:10.1016/S0040-4020(00)00690-6.
- ^ Liu, Na; Song, Wangze; Schienebeck, Casi M.; Zhang, Min; Tang, Weiping (December 2014). "Synthesis of naturally occurring tropones and tropolones". Tetrahedron. 70 (49): 9281–9305. doi:10.1016/j.tet.2014.07.065. PMC 4228802.
- ^ "2,4,6-Cycloheptatrien-1-one, 2-hydroxy-3-(1-methylethyl)-". pubchem.ncbi.nlm.nih.gov. PubChem.
- ^ "Hinokitiol - Product Information" (PDF). www.caymanchem.com. Cayman Chemical.
- ^ "Tetsuo Nozoe (1902−1996)". European Journal of Organic Chemistry. 2004 (4): 899–928. February 2004. doi:10.1002/ejoc.200300579.
- ^ Hendershott, Lynn; Gentilcore, Rita; Ordway, Frederick; Fletcher, James; Donati, Robert (May 1982). "Tropolone: A lipid solubilizing agent for cationic metals". European Journal of Nuclear Medicine. 7 (5). doi:10.1007/BF00256471.
- ^ Bentley, Ronald (2008). "A fresh look at natural tropolonoids". Nat. Prod. Rep. 25 (1): 118–138. doi:10.1039/B711474E.
- ^ "2,4,6-Cycloheptatrien-1-one, 2-hydroxy-3-(1-methylethyl)-". pubchem.ncbi.nlm.nih.gov.
- ^ "Hinokitiol". pubchem.ncbi.nlm.nih.gov.
- ^ "gamma-Thujaplicin". pubchem.ncbi.nlm.nih.gov.
- ^ INAMORI, Yoshihiko; SAKAGAMI, Yoshikazu; MORITA, Yasuhiro; SHIBATA, Mistunobu; SUGIURA, Masaaki; KUMEDA, Yuko; OKABE, Toshihiro; TSUJIBO, Hiroshi; ISHIDA, Nakao (2000). "Antifungal Activity of Hinokitiol-Related Compounds on Wood-Rotting Fungi and Their Insecticidal Activities". Biological & Pharmaceutical Bulletin. 23 (8): 995–997. doi:10.1248/bpb.23.995.
- ^ Pietra, Francesco (August 1973). "Seven-membered conjugated carbo- and heterocyclic compounds and their homoconjugated analogs and metal complexes. Synthesis, biosynthesis, structure, and reactivity". Chemical Reviews. 73 (4): 293–364. doi:10.1021/cr60284a002.
- ^ Aladaileh, Saleem; Rodney, Peters; Nair, Sham V.; Raftos, David A. (December 2007). "Characterization of phenoloxidase activity in Sydney rock oysters (Saccostrea glomerata)". Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology. 148 (4): 470–480. doi:10.1016/j.cbpb.2007.07.089. PMID 17950018.
- ^ Jump up to: a b "Hinokitiol | 499-44-5". www.chemicalbook.com.
- ^ Suzuki, Joichiro; Tokiwa, Tamami; Mochizuki, Maho; Ebisawa, Masato; Nagano, Takatoshi; Yuasa, Mohei; Kanazashi, Mikimoto; Gomi, Kazuhiro; Arai, Takashi (2008). "Effects of a newly designed toothbrush for the application of periodontal disease treatment medicine (HinoporonTM) on the plaque removal and the improvement of gingivitis". Nihon Shishubyo Gakkai Kaishi (Journal of the Japanese Society of Periodontology). 50 (1): 30–38. doi:10.2329/perio.50.030.
- ^ NAKANO, Yasuyuki; MATSUO, Saburo; TANI, Hiroyuki; SASAI, Kazumi; BABA, Eiichiroh (2006). "Therapeutic Effects of β-Thujaplicin Eardrops on Canine Malassezia-Related Otitis Externa". Journal of Veterinary Medical Science. 68 (4): 373–374. doi:10.1292/jvms.68.373. PMID 16679729.
- ^ NAKANO, Yasuyuki; WADA, Makoto; TANI, Hiroyuki; SASAI, Kazumi; BABA, Eiichiroh (2005). "Effects of β-Thujaplicin on Anti-Malassezia pachydermatis Remedy for Canine Otitis Externa". Journal of Veterinary Medical Science. 67 (12): 1243–1247. doi:10.1292/jvms.67.1243. PMID 16397383.
- ^ Aharoni, Y.; Copel, A.; Fallik, E. (June 1993). "Hinokitiol (β‐thujaplicin), for postharvest decay control on 'Galia' melons". New Zealand Journal of Crop and Horticultural Science. 21 (2): 165–169. doi:10.1080/01140671.1993.9513763.
- ^ "The Japan Food chemical Research Faundation". www.ffcr.or.jp.
- ^ L. Brody, Aaron; Strupinsky, E. P.; Kline, Lauri R. (2001). Active Packaging for Food Applications (1 ed.). CRC Press. ISBN 9780367397289.
- ^ MITSUBOSHI, SAORI; OBITSU, RIE; MURAMATSU, KANAKO; FURUBE, KENTARO; YOSHITAKE, SHIGEHIRO; KIUCHI, KAN (2007). "Growth Inhibitory Effect of Shelf Life Extending Agents on Bacillus subtilis IAM 1026". Biocontrol Science. 12 (2): 71–75. doi:10.4265/bio.12.71. PMID 17629249.
- ^ Vanitha, Thiraviam; Thammawong, Manasikan; Umehara, Hitomi; Nakamura, Nobutaka; Shiina, Takeo (3 September 2019). "Effect of hinokitiol impregnated sheets on shelf life and quality of "KEK‐1" tomatoes during storage". Packaging Technology and Science. 32 (12): 641–648. doi:10.1002/pts.2479.
- Tropolones
- Monoterpenes
- Isopropyl compounds
- Non-benzenoid aromatic carbocycles
- Ionophores
- Chelating agents
- Antifungals
- Fungicides
- Antiviral drugs
- Insecticides
- Pesticides
- Skin care
- Oral hygiene
- Antioxidants
- Food additives
