Cannabinoid
| Part of a series on |
| Cannabis |
|---|
 |
|
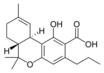
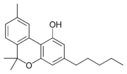
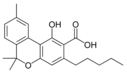
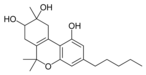
Cannabinoids (/kəˈnæbənɔɪdzˌ ˈkænəbənɔɪdz/) are compounds found in cannabis.[1] The most notable cannabinoid is the phytocannabinoid tetrahydrocannabinol (THC) (Delta9-THC or Delta8-THC), the primary psychoactive compound in cannabis.[2][3] Cannabidiol (CBD) is another major constituent of the plant.[4] At least 113 distinct cannabinoids have been isolated from cannabis.[5]
Classical cannabinoids are structurally related to THC.
Nonclassical cannabinoids (cannabimimetics) include aminoalkylindoles, 1,5-diarylpyrazoles, quinolines, and arylsulfonamides as well as eicosanoids related to endocannabinoids.[2]
Uses[]
Medical uses include the treatment of nausea due to chemotherapy, spasticity, and possibly neuropathic pain.[6] Common side effects include dizziness, sedation, confusion, dissociation, and "feeling high".[6]
Cannabinoid receptors[]
Before the 1980s, cannabinoids were speculated to produce their physiological and behavioral effects via nonspecific interaction with cell membranes, instead of interacting with specific membrane-bound receptors. The discovery of the first cannabinoid receptors in the 1980s helped to resolve this debate.[7] These receptors are common in animals. The cannabinoid receptors are termed CB1 and CB2,[8] with mounting evidence of more.[9] The human brain has more cannabinoid receptors than any other G protein-coupled receptor (GPCR) type.[10]
The Endocannabinoid System (ECS) regulates many functions of the human body. The ECS plays an important role in multiple aspects of neural functions, including the control of movement and motor coordination, learning and memory, emotion and motivation, addictive-like behavior and pain modulation, among others.[11]
Cannabinoid receptor type 1[]
CB1 receptors are found primarily in the brain, more specifically in the basal ganglia and in the limbic system, including the hippocampus[8] and the striatum. They are also found in the cerebellum and in both male and female reproductive systems. CB1 receptors are absent in the medulla oblongata, the part of the brain stem responsible for respiratory and cardiovascular functions. CB1 is also found in the human anterior eye and retina.[12]
Cannabinoid receptor type 2[]
CB2 receptors are predominantly found in the immune system, or immune-derived cells[13][14][15][16] with varying expression patterns. While found only in the peripheral nervous system, a report does indicate that CB2 is expressed by a subpopulation of microglia in the human cerebellum.[17] CB2 receptors appear to be responsible for immunomodulatory[16] and possibly other therapeutic effects of cannabinoid as seen in vitro and in animal models.[15]
Phytocannabinoids[]


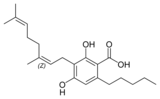
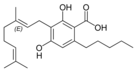
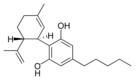
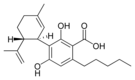
The classical cannabinoids are concentrated in a viscous resin produced in structures known as glandular trichomes. At least 113 different cannabinoids have been isolated from the Cannabis plant[5] To the right, the main classes of cannabinoids from Cannabis are shown.[citation needed]
All classes derive from cannabigerol-type (CBG) compounds and differ mainly in the way this precursor is cyclized.[18] The classical cannabinoids are derived from their respective 2-carboxylic acids (2-COOH) by decarboxylation (catalyzed by heat, light, or alkaline conditions).[19]
Tables[]
By chemical family[]
Decarboxylation temperatures[]
Upon heating, cannabinoid acids decarboxylate to give their psychoactive cannabinoid. For example, Delta-9-tetrahydrocannabinol (THC) is the main psychoactive compound found in cannabis and is responsible for the "high" feeling when consumed.[21] However, cannabis does not naturally contain significant amounts of THC. Instead, tetrahydrocannabinolic acid (THCA) is found naturally in raw and live cannabis and is non-intoxicating.[22] Over time, THCA slowly converts to THC through a process of decarboxylation, but can be sped up with exposure to high temperatures.[23] When heated under conditions of 110°C, decarboxylation generally occurs in 30-45 minutes.[24] This is added to cannabis edibles. When consumed orally, the liver breaks down and metabolizes THC into the more potent 11-hydroxy-THC.[25]
All cannabiniods listed here and their acids are found naturally in the plant to varying degrees.
| Decarboxylation reaction | Temperature |
|---|---|
| CBCA → CBC | |
| → CBCV | |
| CBDA → CBD | |
| → CBDP | |
| → CBDV | |
| → CBE | |
| CBGA → CBG | |
| → | |
| → | |
| → CBL | |
| → CBN | |
| → | |
| → CBV | |
| → delta-8-THC | |
| THCA → THC | 110 °C (230 °F)[26] |
| → THCC | |
| → THCP | |
| → THCV |
Vaporization temperatures[]
Dry-herb vaporizers can be used to inhale cannabis in its flower form.[27] There are 483 identifiable chemical constituents known to exist in the cannabis plant,[28] and at least 85 different cannabinoids have been isolated from the plant.[29] The aromatic terpenoids begin to vaporize at 126.0 °C (258.8 °F),[30] but the more bio-active tetrahydrocannabinol (THC), and other cannabinoids also found in cannabis (often legally sold as cannabinoid isolates) like cannabidiol (CBD), cannabichromene (CBC), cannabigerol (CBG), cannabinol (CBN), do not vaporize until near their respective boiling points.
The cannabinoids listed here are found in the plant but only in trace amounts. However, they have also been extracted and sold as isolates on web shops. 3rd party certification may help ensure buyers to avoid synthetic cannabinoids.
| Cannabinoid | Boiling point |
|---|---|
| CBC | 220 °C (428 °F)[31] |
| CBCV | |
| CBD | 160 °C (320 °F)-180 °C (356 °F)[31] |
| CBDP | |
| CBDV | |
| CBE | |
| CBG | |
| CBL | |
| CBN | 185 °C (365 °F)[31] |
| CBV | |
| delta-8-THC | 175 °C (347 °F)-178 °C (352 °F)[31] |
| THC | 157 °C (315 °F)[31] |
| THCC | |
| THCP | |
| THCV | <220[31] |
Well known cannabinoids[]
The best studied cannabinoids include tetrahydrocannabinol (THC), cannabidiol (CBD) and cannabinol (CBN).
Tetrahydrocannabinol[]
Tetrahydrocannabinol (THC) is the primary psychoactive component of the Cannabis plant. Delta-9-tetrahydrocannabinol (Δ9-THC, THC) and Delta-8-Tetrahydrocannabinol (Δ8-THC), through intracellular CB1 activation, induce anandamide and 2-arachidonoylglycerol synthesis produced naturally in the body and brain[citation needed][dubious ]. These cannabinoids produce the effects associated with cannabis by binding to the CB1 cannabinoid receptors in the brain.[32]
Cannabidiol[]
Cannabidiol (CBD) is non-psychotropic. Evidence shows that the compound counteracts cognitive impairment associated with the use of cannabis.[33] Cannabidiol has little affinity for CB1 and CB2 receptors but acts as an indirect antagonist of cannabinoid agonists.[34] It was found to be an antagonist at the putative new cannabinoid receptor, GPR55, a GPCR expressed in the caudate nucleus and putamen.[35] Cannabidiol has also been shown to act as a 5-HT1A receptor agonist.[36] CBD can interfere with the uptake of adenosine, which plays an important role in biochemical processes, such as energy transfer. It may play a role in promoting sleep and suppressing arousal.[37]
CBD shares a precursor with THC and is the main cannabinoid in CBD-dominant Cannabis strains. CBD has been shown to play a role in preventing the short-term memory loss associated with THC.[38]
There is tentative evidence that CBD has an anti-psychotic effect, but research in this area is limited.[39][33]
Biosynthesis[]
Cannabinoid production starts when an enzyme causes geranyl pyrophosphate and olivetolic acid to combine and form . Next, CBGA is independently converted to either CBG, THCA, CBDA or by four separate synthase, FAD-dependent dehydrogenase enzymes. There is no evidence for enzymatic conversion of CBDA or CBD to THCA or THC. For the propyl homologues (THCVA, CBDVA and CBCVA), there is an analogous pathway that is based on CBGVA from divarinolic acid instead of olivetolic acid.
Double bond position[]
In addition, each of the compounds above may be in different forms depending on the position of the double bond in the alicyclic carbon ring. There is potential for confusion because there are different numbering systems used to describe the position of this double bond. Under the dibenzopyran numbering system widely used today, the major form of THC is called Δ9-THC, while the minor form is called Δ8-THC. Under the alternate terpene numbering system, these same compounds are called Δ1-THC and Δ6-THC, respectively.
Length[]
Most classical cannabinoids are 21-carbon compounds. However, some do not follow this rule, primarily because of variation in the length of the side-chain attached to the aromatic ring. In THC, CBD, and CBN, this side-chain is a pentyl (5-carbon) chain. In the most common homologue, the pentyl chain is replaced with a propyl (3-carbon) chain. Cannabinoids with the propyl side chain are named using the suffix varin and are designated THCV, CBDV, or CBNV, while those with the heptyl side chain are named using the suffix phorol and are designated THCP and CBDP.
Cannabinoids in other plants[]
Phytocannabinoids are known to occur in several plant species besides cannabis. These include Echinacea purpurea, Echinacea angustifolia, Acmella oleracea, Helichrysum umbraculigerum, and Radula marginata.[40] The best-known cannabinoids that are not derived from Cannabis are the lipophilic alkamides (alkylamides) from Echinacea species, most notably the cis/trans isomers dodeca-2E,4E,8Z,10E/Z-tetraenoic-acid-isobutylamide.[40] At least 25 different have been identified, and some of them have shown affinities to the CB2-receptor.[41][42] In some Echinacea species, cannabinoids are found throughout the plant structure, but are most concentrated in the roots and flowers.[43][44] Yangonin found in the Kava plant has significant affinity to the CB1 receptor.[45] Tea (Camellia sinensis) catechins have an affinity for human cannabinoid receptors.[46] A widespread dietary terpene, beta-caryophyllene, a component from the essential oil of cannabis and other medicinal plants, has also been identified as a selective agonist of peripheral CB2-receptors, in vivo.[47] Black truffles contain anandamide.[48] Perrottetinene, a moderately psychoactive cannabinoid,[49] has been isolated from different Radula varieties.
Most of the phytocannabinoids are nearly insoluble in water but are soluble in lipids, alcohols, and other non-polar organic solvents.
Cannabis plant profile[]
Cannabis plants can exhibit wide variation in the quantity and type of cannabinoids they produce. The mixture of cannabinoids produced by a plant is known as the plant's cannabinoid profile. Selective breeding has been used to control the genetics of plants and modify the cannabinoid profile. For example, strains that are used as fiber (commonly called hemp) are bred such that they are low in psychoactive chemicals like THC. Strains used in medicine are often bred for high CBD content, and strains used for recreational purposes are usually bred for high THC content or for a specific chemical balance.
Quantitative analysis of a plant's cannabinoid profile is often determined by gas chromatography (GC), or more reliably by gas chromatography combined with mass spectrometry (GC/MS). Liquid chromatography (LC) techniques are also possible and, unlike GC methods, can differentiate between the acid and neutral forms of the cannabinoids. There have been systematic attempts to monitor the cannabinoid profile of cannabis over time, but their accuracy is impeded by the illegal status of the plant in many countries.
Pharmacology[]
Cannabinoids can be administered by smoking, vaporizing, oral ingestion, transdermal patch, intravenous injection, sublingual absorption, or rectal suppository. Once in the body, most cannabinoids are metabolized in the liver, especially by cytochrome P450 mixed-function oxidases, mainly CYP 2C9.[50] Thus supplementing with CYP 2C9 inhibitors leads to extended intoxication.[50]
Some is also stored in fat in addition to being metabolized in the liver. Δ9-THC is metabolized to 11-hydroxy-Δ9-THC, which is then metabolized to 9-carboxy-THC.[51] Some cannabis metabolites can be detected in the body several weeks after administration. These metabolites are the chemicals recognized by common antibody-based "drug tests"; in the case of THC or others, these loads do not represent intoxication (compare to ethanol breath tests that measure instantaneous blood alcohol levels), but an integration of past consumption over an approximately month-long window. This is because they are fat-soluble, lipophilic molecules that accumulate in fatty tissues.[52]
Research shows the effect of cannabinoids might be modulated by aromatic compounds produced by the cannabis plant, called terpenes. This interaction would lead to the entourage effect.[53]
Cannabinoid-based pharmaceuticals[]
Nabiximols (brand name Sativex) is an aerosolized mist for oral administration containing a near 1:1 ratio of CBD and THC.[54] Also included are minor cannabinoids and terpenoids, ethanol and propylene glycol excipients, and peppermint flavoring.[55] The drug, made by GW Pharmaceuticals, was first approved by Canadian authorities in 2005 to alleviate pain associated with multiple sclerosis, making it the first cannabis-based medicine. It is marketed by Bayer in Canada.[56] Sativex has been approved in 25 countries; clinical trials are underway in the United States to gain FDA approval.[57] In 2007, it was approved for treatment of cancer pain.[55] In Phase III trials, the most common adverse effects were dizziness, drowsiness and disorientation; 12% of subjects stopped taking the drug because of the side effects.[58]
Dronabinol (brand name Marinol) is a THC drug used to treat poor appetite, nausea, and sleep apnea.[59] It is approved by the FDA for treating HIV/AIDS induced anorexia and chemotherapy induced nausea and vomiting.[60][61][62]
The CBD drug Epidiolex has been approved by the Food and Drug Administration for treatment of two rare and severe forms of epilepsy,[63] Dravet and Lennox-Gastaut syndromes.[64]
Separation[]
Cannabinoids can be separated from the plant by extraction with organic solvents. Hydrocarbons and alcohols are often used as solvents. However, these solvents are flammable and many are toxic.[65] Butane may be used, which evaporates extremely quickly. Supercritical solvent extraction with carbon dioxide is an alternative technique. Once extracted, isolated components can be separated using wiped film vacuum distillation or other distillation techniques.[66] Also, techniques such as SPE or SPME are found useful in the extraction of these compounds.[67]
History[]
The first discovery of an individual cannabinoid was made, when British chemist Robert S. Cahn reported the partial structure of Cannabinol (CBN), which he later identified as fully formed in 1940.
Two years later, in 1942,[68] American chemist, Roger Adams, made history when he discovered Cannabidiol (CBD).[69] Progressing from Adams research, in 1963[70] Israeli professor Raphael Mechoulam[71] later identified the stereochemistry of CBD. The following year, in 1964,[70] Mechoulam and his team identified the stereochemistry of Tetrahydrocannabinol (THC).[citation needed]
Due to molecular similarity and ease of synthetic conversion, CBD was originally believed to be a natural precursor to THC. However, it is now known that CBD and THC are produced independently in the cannabis plant from the precursor CBG.[citation needed]
Endocannabinoids[]

Endocannabinoids are substances produced from within the body that activate cannabinoid receptors. After the discovery of the first cannabinoid receptor in 1988, scientists began searching for an endogenous ligand for the receptor.[7][72]
Types of endocannabinoid ligands[]
Arachidonoylethanolamine (Anandamide or AEA)[]
Anandamide was the first such compound identified as arachidonoyl ethanolamine. The name is derived from the Sanskrit word for bliss and -amide. It has a pharmacology similar to THC, although its structure is quite different. Anandamide binds to the central (CB1) and, to a lesser extent, peripheral (CB2) cannabinoid receptors, where it acts as a partial agonist. Anandamide is about as potent as THC at the CB1 receptor.[73] Anandamide is found in nearly all tissues in a wide range of animals.[74] Anandamide has also been found in plants, including small amounts in chocolate.[75]
Two analogs of anandamide, 7,10,13,16-docosatetraenoylethanolamide and homo-γ-linolenoylethanolamine, have similar pharmacology. All of these compounds are members of a family of signalling lipids called N-acylethanolamines, which also includes the noncannabimimetic palmitoylethanolamide and oleoylethanolamide, which possess anti-inflammatory and anorexigenic effects, respectively. Many N-acylethanolamines have also been identified in plant seeds[76] and in molluscs.[77]
2-Arachidonoylglycerol (2-AG)[]
Another endocannabinoid, 2-arachidonoylglycerol, binds to both the CB1 and CB2 receptors with similar affinity, acting as a full agonist at both.[73] 2-AG is present at significantly higher concentrations in the brain than anandamide,[78] and there is some controversy over whether 2-AG rather than anandamide is chiefly responsible for endocannabinoid signalling in vivo.[8] In particular, one in vitro study suggests that 2-AG is capable of stimulating higher G-protein activation than anandamide, although the physiological implications of this finding are not yet known.[79]
2-Arachidonyl glyceryl ether (noladin ether)[]
In 2001, a third, ether-type endocannabinoid, 2-arachidonyl glyceryl ether (noladin ether), was isolated from porcine brain.[80] Prior to this discovery, it had been synthesized as a stable analog of 2-AG; indeed, some controversy remains over its classification as an endocannabinoid, as another group failed to detect the substance at "any appreciable amount" in the brains of several different mammalian species.[81] It binds to the CB1 cannabinoid receptor (Ki = 21.2 nmol/L) and causes sedation, hypothermia, intestinal immobility, and mild antinociception in mice. It binds primarily to the CB1 receptor, and only weakly to the CB2 receptor.[73]
N-Arachidonoyl dopamine (NADA)[]
Discovered in 2000, NADA preferentially binds to the CB1 receptor.[82] Like anandamide, NADA is also an agonist for the vanilloid receptor subtype 1 (TRPV1), a member of the vanilloid receptor family.[83][84]
Virodhamine (OAE)[]
A fifth endocannabinoid, virodhamine, or O-arachidonoyl-ethanolamine (OAE), was discovered in June 2002. Although it is a full agonist at CB2 and a partial agonist at CB1, it behaves as a CB1 antagonist in vivo. In rats, virodhamine was found to be present at comparable or slightly lower concentrations than anandamide in the brain, but 2- to 9-fold higher concentrations peripherally.[85]
Lysophosphatidylinositol (LPI)[]
Lysophosphatidylinositol is the endogenous ligand to novel endocannabinoid receptor GPR55, making it a strong contender as the sixth endocannabinoid.[86]
Function[]
This section needs additional citations for verification. (October 2018) |
Endocannabinoids serve as intercellular 'lipid messengers',[87] signaling molecules that are released from one cell and activating the cannabinoid receptors present on other nearby cells. Although in this intercellular signaling role they are similar to the well-known monoamine neurotransmitters such as dopamine, endocannabinoids differ in numerous ways from them. For instance, they are used in retrograde signaling between neurons.[88] Furthermore, endocannabinoids are lipophilic molecules that are not very soluble in water. They are not stored in vesicles and exist as integral constituents of the membrane bilayers that make up cells. They are believed to be synthesized 'on-demand' rather than made and stored for later use.
As hydrophobic molecules, endocannabinoids cannot travel unaided for long distances in the aqueous medium surrounding the cells from which they are released and therefore act locally on nearby target cells. Hence, although emanating diffusely from their source cells, they have much more restricted spheres of influence than do hormones, which can affect cells throughout the body.
The mechanisms and enzymes underlying the biosynthesis of endocannabinoids remain elusive and continue to be an area of active research.
The endocannabinoid 2-AG has been found in bovine and human maternal milk.[89]
A review by Matties et al. (1994) summed up the phenomenon of gustatory enhancement by certain cannabinoids.[90] The sweet receptor (Tlc1) is stimulated by indirectly increasing its expression and suppressing the activity of leptin, the Tlc1 antagonist. It is proposed that the competition of leptin and cannabinoids for Tlc1 is implicated in energy homeostasis.[91]
Retrograde signal[]
Conventional neurotransmitters are released from a ‘presynaptic’ cell and activate appropriate receptors on a ‘postsynaptic’ cell, where presynaptic and postsynaptic designate the sending and receiving sides of a synapse, respectively. Endocannabinoids, on the other hand, are described as retrograde transmitters because they most commonly travel ‘backward’ against the usual synaptic transmitter flow. They are, in effect, released from the postsynaptic cell and act on the presynaptic cell, where the target receptors are densely concentrated on axonal terminals in the zones from which conventional neurotransmitters are released. Activation of cannabinoid receptors temporarily reduces the amount of conventional neurotransmitter released. This endocannabinoid-mediated system permits the postsynaptic cell to control its own incoming synaptic traffic. The ultimate effect on the endocannabinoid-releasing cell depends on the nature of the conventional transmitter being controlled. For instance, when the release of the inhibitory transmitter GABA is reduced, the net effect is an increase in the excitability of the endocannabinoid-releasing cell. On the converse, when release of the excitatory neurotransmitter glutamate is reduced, the net effect is a decrease in the excitability of the endocannabinoid-releasing cell.[92][citation needed]
"Runner's high"[]
The runner's high, the slight euphoria that sometimes accompanies aerobic exercise, has often been attributed to the release of endorphins, but newer research suggests that it might be due to endocannabinoids instead.[93]
Synthetic cannabinoids[]
Historically, laboratory synthesis of cannabinoids was often based on the structure of herbal cannabinoids, and a large number of analogs have been produced and tested, especially in a group led by Roger Adams as early as 1941 and later in a group led by Raphael Mechoulam. Newer compounds are no longer related to natural cannabinoids or are based on the structure of the endogenous cannabinoids.[94]
Synthetic cannabinoids are particularly useful in experiments to determine the relationship between the structure and activity of cannabinoid compounds, by making systematic, incremental modifications of cannabinoid molecules.[95]
When synthetic cannabinoids are used recreationally, they present significant health dangers to users.[96] In the period of 2012 through 2014, over 10,000 contacts to poison control centers in the United States were related to use of synthetic cannabinoids.[96]
Medications containing natural or synthetic cannabinoids or cannabinoid analogs:
- Dronabinol (Marinol), is Δ9-tetrahydrocannabinol (THC), used as an appetite stimulant, anti-emetic, and analgesic
- Nabilone (Cesamet, Canemes), a synthetic cannabinoid and an analog of Marinol. It is Schedule II unlike Marinol, which is Schedule III
- Rimonabant (SR141716), a selective cannabinoid (CB1) receptor inverse agonist once used as an anti-obesity drug under the proprietary name Acomplia. It was also used for smoking cessation
Other notable synthetic cannabinoids include:
- JWH-018, a potent synthetic cannabinoid agonist discovered by John W. Huffman at Clemson University. It was often sold in legal smoke blends collectively known as "spice". Several countries and states have moved to ban it legally.
- JWH-073
- CP-55940, produced in 1974, this synthetic cannabinoid receptor agonist is many times more potent than THC.
- Dimethylheptylpyran
- HU-210, about 100 times as potent as THC[97]
- HU-211, a synthetic cannabinoid derived drug that acts on NMDA instead of endocannabinoid system
- HU-331 a potential anti-cancer drug derived from cannabidiol that specifically inhibits topoisomerase II.
- SR144528, a CB2 receptor antagonist/ inverse agonist[98]
- WIN 55,212-2, a potent cannabinoid receptor agonist
- JWH-133, a potent selective CB2 receptor agonist
- Levonantradol (Nantrodolum), an anti-emetic and analgesic but not currently in use in medicine
- AM-2201, a potent cannabinoid receptor agonist
See also[]
- Cancer and nausea § Cannabinoid
- Cannabinoid receptor antagonist
- Endocannabinoid enhancer
- Endocannabinoid reuptake inhibitor
References[]
- ^ "Marijuana, also called: Cannabis, Ganja, Grass, Hash, Pot, Weed". Medline Plus. 3 July 2017.
- ^ Jump up to: a b Lambert DM, Fowler CJ (August 2005). "The endocannabinoid system: drug targets, lead compounds, and potential therapeutic applications". Journal of Medicinal Chemistry. 48 (16): 5059–87. doi:10.1021/jm058183t. PMID 16078824.
- ^ Pertwee, Roger, ed. (2005). Cannabinoids. Springer-Verlag. p. 2. ISBN 978-3-540-22565-2.
- ^ "Bulletin on Narcotics – 1962 Issue 3 – 004". UNODC (United Nations Office of Drugs and Crime). 1962-01-01. Retrieved 2014-01-15.
- ^ Jump up to: a b Aizpurua-Olaizola O, Soydaner U, Öztürk E, Schibano D, Simsir Y, Navarro P, Etxebarria N, Usobiaga A (February 2016). "Evolution of the Cannabinoid and Terpene Content during the Growth of Cannabis sativa Plants from Different Chemotypes". Journal of Natural Products. 79 (2): 324–31. doi:10.1021/acs.jnatprod.5b00949. PMID 26836472.
- ^ Jump up to: a b Allan GM, Finley CR, Ton J, Perry D, Ramji J, Crawford K, Lindblad AJ, Korownyk C, Kolber MR (February 2018). "Systematic review of systematic reviews for medical cannabinoids: Pain, nausea and vomiting, spasticity, and harms". Canadian Family Physician. 64 (2): e78–e94. PMC 5964405. PMID 29449262.
- ^ Jump up to: a b Devane WA, Dysarz FA, Johnson MR, Melvin LS, Howlett AC (November 1988). "Determination and characterization of a cannabinoid receptor in rat brain". Molecular Pharmacology. 34 (5): 605–13. PMID 2848184.
- ^ Jump up to: a b c Pacher P, Bátkai S, Kunos G (September 2006). "The endocannabinoid system as an emerging target of pharmacotherapy". Pharmacological Reviews. 58 (3): 389–462. doi:10.1124/pr.58.3.2. PMC 2241751. PMID 16968947.
- ^ Begg M, Pacher P, Bátkai S, Osei-Hyiaman D, Offertáler L, Mo FM, Liu J, Kunos G (May 2005). "Evidence for novel cannabinoid receptors". Pharmacology & Therapeutics. 106 (2): 133–45. doi:10.1016/j.pharmthera.2004.11.005. PMID 15866316.
- ^ Boron WF, Boulpaep EL, eds. (2009). Medical Physiology: A Cellular and Molecular Approach. Saunders. p. 331. ISBN 978-1-4160-3115-4.
- ^ "Endocannabinoid System - an overview | ScienceDirect Topics". www.sciencedirect.com. Retrieved 2021-05-28.
- ^ Straiker AJ, Maguire G, Mackie K, Lindsey J (September 1999). "Localization of cannabinoid CB1 receptors in the human anterior eye and retina". Investigative Ophthalmology & Visual Science. 40 (10): 2442–8. PMID 10476817.
- ^ Marchand, J.; Bord, A.; Pénarier, G.; Lauré, F.; Carayon, P.; Casellas, P. (1999-03-01). "Quantitative method to determine mRNA levels by reverse transcriptase-polymerase chain reaction from leukocyte subsets purified by fluorescence-activated cell sorting: application to peripheral cannabinoid receptors". Cytometry. 35 (3): 227–234. doi:10.1002/(SICI)1097-0320(19990301)35:3<227::AID-CYTO5>3.0.CO;2-4. ISSN 0196-4763. PMID 10082303.
- ^ Galiègue, S.; Mary, S.; Marchand, J.; Dussossoy, D.; Carrière, D.; Carayon, P.; Bouaboula, M.; Shire, D.; Le Fur, G.; Casellas, P. (1995-08-15). "Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations". European Journal of Biochemistry. 232 (1): 54–61. doi:10.1111/j.1432-1033.1995.tb20780.x. ISSN 0014-2956. PMID 7556170.
- ^ Jump up to: a b Pacher P, Mechoulam R (April 2011). "Is lipid signaling through cannabinoid 2 receptors part of a protective system?". Progress in Lipid Research. 50 (2): 193–211. doi:10.1016/j.plipres.2011.01.001. PMC 3062638. PMID 21295074.
- ^ Jump up to: a b Saroz, Yurii; Kho, Dan T.; Glass, Michelle; Graham, Euan Scott; Grimsey, Natasha Lillia (2019-10-19). "Cannabinoid Receptor 2 (CB 2 ) Signals via G-alpha-s and Induces IL-6 and IL-10 Cytokine Secretion in Human Primary Leukocytes". ACS Pharmacology & Translational Science. 2 (6): 414–428. doi:10.1021/acsptsci.9b00049. ISSN 2575-9108. PMC 7088898. PMID 32259074.
- ^ Núñez E, Benito C, Pazos MR, Barbachano A, Fajardo O, González S, Tolón RM, Romero J (September 2004). "Cannabinoid CB2 receptors are expressed by perivascular microglial cells in the human brain: an immunohistochemical study". Synapse. 53 (4): 208–13. doi:10.1002/syn.20050. PMID 15266552. S2CID 40738073.
- ^ Fellermeier M, Eisenreich W, Bacher A, Zenk MH (March 2001). "Biosynthesis of cannabinoids. Incorporation experiments with (13)C-labeled glucoses". European Journal of Biochemistry. 268 (6): 1596–604. doi:10.1046/j.1432-1327.2001.02030.x. PMID 11248677.
- ^ Patentdocs. Patent application title: Controlled cannabis decarboxylation. US Patent application number: 20120046352. Retrieved 28 December 2013
- ^ (PDF) https://leg.mt.gov/content/Committees/Interim/2009_2010/Children_Family/Emerging-Issue/mmga-presentation-cannabinoid-table-aug2010.pdf. Missing or empty
|title=(help) - ^ Weedmaps Editors. "What is Tetrohydrocannabinol? | Delta 9 THC Definition by Weedmaps". Weedmaps. Retrieved 2021-04-10.
- ^ Rahn B (17 March 2015). "What is THCA and what are the benefits of this cannabinoid?". Leafly. Retrieved 2021-04-10.
- ^ Bennett P (30 April 2016). "What Is Decarboxylation, and Why Does Your Cannabis Need It?". Leafly. Retrieved 2019-08-28.
- ^ "Why Decarboxylating Your Cannabis Is So Important - RQS Blog". www.royalqueenseeds.com. Retrieved 2021-04-10.
- ^ Weedmaps Editors. "What Is 11-hydroxy-THC? 11-hydroxy-THC Definition By Weedmaps". Weedmaps. Retrieved 2021-04-10.
- ^ Wang, M; Wang, YH; Avula, B; Radwan, MM; Wanas, AS; van Antwerp, J; Parcher, JF; ElSohly, MA; Khan, IA (2016). "Decarboxylation Study of Acidic Cannabinoids: A Novel Approach Using Ultra-High-Performance Supercritical Fluid Chromatography/Photodiode Array-Mass Spectrometry". Cannabis and cannabinoid research. 1 (1): 262–271. doi:10.1089/can.2016.0020. PMC 5549281. PMID 28861498.
- ^ "Benefits of Using a Dry Herb Vaporizer". KingPenVapes. July 21, 2016. Retrieved June 2, 2018.
- ^ "What chemicals are in marijuana and its byproducts?". ProCon.org. 2009. Retrieved 2013-01-13.
- ^ El-Alfy; Abir T; et al. (Jun 2010). "Antidepressant-like effect of delta-9-tetrahydrocannabinol and other cannabinoids isolated from Cannabis sativa L". Pharmacology Biochemistry and Behavior. 95 (4): 434–42. doi:10.1016/j.pbb.2010.03.004. PMC 2866040. PMID 20332000.
- ^ "Methods of Medicating with Marijuana". evaluationtoday.com. Retrieved 10 February 2014.
- ^ Jump up to: a b c d e f "Phytocannabinoid Boiling Points" (PDF). projectcbd.org. Retrieved 20 August 2021.
- ^ How does marijuana produce its effects?, drugabuse.gov
- ^ Jump up to: a b Iseger TA, Bossong MG (March 2015). "A systematic review of the antipsychotic properties of cannabidiol in humans". Schizophrenia Research. 162 (1–3): 153–61. doi:10.1016/j.schres.2015.01.033. PMID 25667194. S2CID 3745655.
- ^ Mechoulam R, Peters M, Murillo-Rodriguez E, Hanus LO (August 2007). "Cannabidiol--recent advances". Chemistry & Biodiversity. 4 (8): 1678–92. doi:10.1002/cbdv.200790147. PMID 17712814. S2CID 3689072.
- ^ Ryberg E, Larsson N, Sjögren S, Hjorth S, Hermansson NO, Leonova J, Elebring T, Nilsson K, Drmota T, Greasley PJ (December 2007). "The orphan receptor GPR55 is a novel cannabinoid receptor". British Journal of Pharmacology. 152 (7): 1092–101. doi:10.1038/sj.bjp.0707460. PMC 2095107. PMID 17876302.
- ^ Russo EB, Burnett A, Hall B, Parker KK (August 2005). "Agonistic properties of cannabidiol at 5-HT1a receptors". Neurochemical Research. 30 (8): 1037–43. doi:10.1007/s11064-005-6978-1. PMID 16258853. S2CID 207222631.
- ^ Campos AC, Moreira FA, Gomes FV, Del Bel EA, Guimarães FS (December 2012). "Multiple mechanisms involved in the large-spectrum therapeutic potential of cannabidiol in psychiatric disorders". Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 367 (1607): 3364–78. doi:10.1098/rstb.2011.0389. PMC 3481531. PMID 23108553.
- ^ Frood, Arron (2010). "Key ingredient staves off marijuana memory loss". Nature. doi:10.1038/news.2010.508. Retrieved 6 October 2015.
- ^ Leweke FM, Mueller JK, Lange B, Rohleder C (April 2016). "Therapeutic Potential of Cannabinoids in Psychosis". Biological Psychiatry. 79 (7): 604–12. doi:10.1016/j.biopsych.2015.11.018. PMID 26852073. S2CID 24160677.
- ^ Jump up to: a b Woelkart K, Salo-Ahen OM, Bauer R (2008). "CB receptor ligands from plants". Current Topics in Medicinal Chemistry. 8 (3): 173–86. doi:10.2174/156802608783498023. PMID 18289087.
- ^ Bauer R, Remiger P (August 1989). "TLC and HPLC Analysis of Alkamides in Echinacea Drugs1,2". Planta Medica. 55 (4): 367–71. doi:10.1055/s-2006-962030. PMID 17262436.
- ^ Raduner S, Majewska A, Chen JZ, Xie XQ, Hamon J, Faller B, Altmann KH, Gertsch J (May 2006). "Alkylamides from Echinacea are a new class of cannabinomimetics. Cannabinoid type 2 receptor-dependent and -independent immunomodulatory effects". The Journal of Biological Chemistry. 281 (20): 14192–206. doi:10.1074/jbc.M601074200. PMID 16547349.
- ^ Perry NB, van Klink JW, Burgess EJ, Parmenter GA (February 1997). "Alkamide levels in Echinacea purpurea: a rapid analytical method revealing differences among roots, rhizomes, stems, leaves and flowers". Planta Medica. 63 (1): 58–62. doi:10.1055/s-2006-957605. PMID 17252329.
- ^ He X, Lin L, Bernart MW, Lian L (1998). "Analysis of alkamides in roots and achenes of Echinacea purpurea by liquid chromatography–electrospray mass spectrometry". Journal of Chromatography A. 815 (2): 205–11. doi:10.1016/S0021-9673(98)00447-6.
- ^ Ligresti A, Villano R, Allarà M, Ujváry I, Di Marzo V (August 2012). "Kavalactones and the endocannabinoid system: the plant-derived yangonin is a novel CB₁ receptor ligand". Pharmacological Research. 66 (2): 163–9. doi:10.1016/j.phrs.2012.04.003. PMID 22525682.
- ^ Korte G, Dreiseitel A, Schreier P, Oehme A, Locher S, Geiger S, Heilmann J, Sand PG (January 2010). "Tea catechins' affinity for human cannabinoid receptors". Phytomedicine. 17 (1): 19–22. doi:10.1016/j.phymed.2009.10.001. PMID 19897346.
- ^ Gertsch J, Leonti M, Raduner S, Racz I, Chen JZ, Xie XQ, Altmann KH, Karsak M, Zimmer A (July 2008). "Beta-caryophyllene is a dietary cannabinoid". Proceedings of the National Academy of Sciences of the United States of America. 105 (26): 9099–104. Bibcode:2008PNAS..105.9099G. doi:10.1073/pnas.0803601105. PMC 2449371. PMID 18574142.
- ^ Pacioni G, Rapino C, Zarivi O, Falconi A, Leonardi M, Battista N, Colafarina S, Sergi M, Bonfigli A, Miranda M, Barsacchi D, Maccarrone M (February 2015). "Truffles contain endocannabinoid metabolic enzymes and anandamide". Phytochemistry. 110: 104–10. doi:10.1016/j.phytochem.2014.11.012. PMID 25433633.
- ^ Chicca, A.; Schafroth, M. A.; Reynoso-Moreno, I.; Erni, R.; Petrucci, V.; Carreira, E. M.; Gertsch, J. (2018-10-01). "Uncovering the psychoactivity of a cannabinoid from liverworts associated with a legal high". Science Advances. 4 (10): eaat2166. Bibcode:2018SciA....4.2166C. doi:10.1126/sciadv.aat2166. ISSN 2375-2548. PMC 6200358. PMID 30397641.
- ^ Jump up to: a b Stout SM, Cimino NM (February 2014). "Exogenous cannabinoids as substrates, inhibitors, and inducers of human drug metabolizing enzymes: a systematic review". Drug Metabolism Reviews. 46 (1): 86–95. doi:10.3109/03602532.2013.849268. PMID 24160757. S2CID 29133059.
- ^ Aizpurua-Olaizola O, Zarandona I, Ortiz L, Navarro P, Etxebarria N, Usobiaga A (April 2017). "Simultaneous quantification of major cannabinoids and metabolites in human urine and plasma by HPLC-MS/MS and enzyme-alkaline hydrolysis". Drug Testing and Analysis. 9 (4): 626–633. doi:10.1002/dta.1998. PMID 27341312.
- ^ Ashton CH (February 2001). "Pharmacology and effects of cannabis: a brief review". The British Journal of Psychiatry. 178 (2): 101–6. doi:10.1192/bjp.178.2.101. PMID 11157422.
Because they are extremely lipid soluble, cannabinoids accumulate in fatty tissues, reaching peak concentrations in 4-5 days. They are then slowly released back into other body compartments, including the brain. They are then slowly released back into other body compartments, including the brain. Because of the sequestration in fat, the tissue elimination half-life of THC is about 7 days, and complete elimination of a single dose may take up to 30 days.
- ^ Russo, Ethan (2011). "Taming THC: potential cannabis synergy and phytocannabinoid-terpenoid entourage effects". British Journal of Pharmacology. 163 (7): 1344–1364. doi:10.1111/j.1476-5381.2011.01238.x. PMC 3165946. PMID 21749363.
- ^ Keating, Gillian M. (14 March 2017). "Delta-9-Tetrahydrocannabinol/Cannabidiol Oromucosal Spray (Sativex®): A Review in Multiple Sclerosis-Related Spasticity". Drugs. 77 (5): 563–574. doi:10.1007/s40265-017-0720-6. PMID 28293911. S2CID 2884550.
- ^ Jump up to: a b Russo, E. B. (2008). "Cannabinoids in the management of difficult to treat pain". Therapeutics and Clinical Risk Management. 4 (1): 245–259. doi:10.2147/TCRM.S1928. PMC 2503660. PMID 18728714.
- ^ Cooper, Rachel (21 June 2010). "GW Pharmaceuticals launches world's first prescription cannabis drug in Britain". Retrieved 29 November 2018.
- ^ "3 prescription drugs that come from marijuana". USA Today. Retrieved 30 November 2018.
- ^ Schubert-Zsilavecz, M, Wurglics, M, Neue Arzneimittel 2011/2012 (in German)
- ^ "Can Dronabinol Help Treat Sleep Apnea? | HealthCentral". www.healthcentral.com. Retrieved 2018-11-04.
- ^ "Marinol (Dronabinol)" (PDF). US Food and Drug Administration. September 2004. Retrieved 14 January 2018.
- ^ "Cannabis and Cannabinoids". National Cancer Institute. 24 October 2011. Retrieved 12 January 2014.
- ^ Badowski ME (September 2017). "A review of oral cannabinoids and medical marijuana for the treatment of chemotherapy-induced nausea and vomiting: a focus on pharmacokinetic variability and pharmacodynamics". Cancer Chemotherapy and Pharmacology. 80 (3): 441–449. doi:10.1007/s00280-017-3387-5. PMC 5573753. PMID 28780725.
- ^ "FDA approves first drug comprised of an active ingredient derived from marijuana to treat rare, severe forms of epilepsy". US Food and Drug Administration. 25 June 2018. Retrieved 25 June 2018.
- ^ Scutti, Susan (25 June 2018). "FDA approves first cannabis-based drug". CNN.
- ^ Romano L, Hazekamp A (2013). "Cannabis Oil: chemical evaluation of an upcoming cannabis-based medicine" (PDF). Cannabinoids. 7 (1): 1–11.
- ^ Rovetto L, Aieta N (November 2017). "Supercritical carbon dioxide extraction of cannabinoids from Cannabis sativa L.". The Journal of Supercritical Fluids. 129: 16–27. doi:10.1016/j.supflu.2017.03.014.
- ^ Jain R, Singh R (2016). "Microextraction techniques for analysis of cannabinoids". TrAC Trends in Analytical Chemistry. 80: 156–166. doi:10.1016/j.trac.2016.03.012.
- ^ Weinberg, Bill (Fall 2018). "U.S. Chemist Roger Adams Isolated CBD 75 Years Ago". Freedom Leaf (34 ed.). Freedom Leaf. Retrieved 2019-03-16 – via Issuu.com.
- ^ Cadena, Aaron (2019-03-08). "The History Of CBD - A Brief Overview". CBD Origin. CBDOrigin.com. Retrieved 2019-03-16.
- ^ Jump up to: a b Pertwee, Roger G (January 2006). "Cannabinoid pharmacology: the first 66 years". British Journal of Pharmacology. 147 (Suppl 1): S163–S171. doi:10.1038/sj.bjp.0706406. ISSN 0007-1188. PMC 1760722. PMID 16402100.
- ^ Mechoulam, Raphael. "Raphael Mechoulam Ph.D." cannabinoids.huji.ac.il (Biography). The Hebrew University of Jerusalem. Retrieved 2019-03-16.
- ^ Katona I, Freund TF (2012). "Multiple functions of endocannabinoid signaling in the brain". Annual Review of Neuroscience. 35: 529–58. doi:10.1146/annurev-neuro-062111-150420. PMC 4273654. PMID 22524785.
- ^ Jump up to: a b c Grotenhermen, Franjo (2005). "Cannabinoids". Current Drug Targets. CNS & Neurological Disorders. 4 (5): 507–530. doi:10.2174/156800705774322111. PMID 16266285.
- ^ Martin BR, Mechoulam R, Razdan RK (1999). "Discovery and characterization of endogenous cannabinoids". Life Sciences. 65 (6–7): 573–95. doi:10.1016/S0024-3205(99)00281-7. PMID 10462059.
- ^ di Tomaso E, Beltramo M, Piomelli D (August 1996). "Brain cannabinoids in chocolate". Nature (Submitted manuscript). 382 (6593): 677–8. Bibcode:1996Natur.382..677D. doi:10.1038/382677a0. PMID 8751435. S2CID 4325706.
- ^ Chapman KD, Venables B, Markovic R, Bettinger C (August 1999). "N-Acylethanolamines in seeds. Quantification Of molecular species and their degradation upon imbibition". Plant Physiology. 120 (4): 1157–64. doi:10.1104/pp.120.4.1157. PMC 59349. PMID 10444099.
- ^ Sepe N, De Petrocellis L, Montanaro F, Cimino G, Di Marzo V (January 1998). "Bioactive long chain N-acylethanolamines in five species of edible bivalve molluscs. Possible implications for mollusc physiology and sea food industry". Biochimica et Biophysica Acta. 1389 (2): 101–11. doi:10.1016/S0005-2760(97)00132-X. PMID 9461251.
- ^ Stella N, Schweitzer P, Piomelli D (August 1997). "A second endogenous cannabinoid that modulates long-term potentiation". Nature (Submitted manuscript). 388 (6644): 773–8. Bibcode:1997Natur.388..773S. doi:10.1038/42015. PMID 9285589. S2CID 4422311.
- ^ Savinainen JR, Järvinen T, Laine K, Laitinen JT (October 2001). "Despite substantial degradation, 2-arachidonoylglycerol is a potent full efficacy agonist mediating CB(1) receptor-dependent G-protein activation in rat cerebellar membranes". British Journal of Pharmacology. 134 (3): 664–72. doi:10.1038/sj.bjp.0704297. PMC 1572991. PMID 11588122.
- ^ Hanus L, Abu-Lafi S, Fride E, Breuer A, Vogel Z, Shalev DE, Kustanovich I, Mechoulam R (March 2001). "2-arachidonyl glyceryl ether, an endogenous agonist of the cannabinoid CB1 receptor". Proceedings of the National Academy of Sciences of the United States of America. 98 (7): 3662–5. Bibcode:2001PNAS...98.3662H. doi:10.1073/pnas.061029898. PMC 31108. PMID 11259648.
- ^ Oka S, Tsuchie A, Tokumura A, Muramatsu M, Suhara Y, Takayama H, Waku K, Sugiura T (June 2003). "Ether-linked analogue of 2-arachidonoylglycerol (noladin ether) was not detected in the brains of various mammalian species". Journal of Neurochemistry. 85 (6): 1374–81. doi:10.1046/j.1471-4159.2003.01804.x. PMID 12787057. S2CID 39905742.
- ^ Bisogno T, Melck D, Gretskaya NM, Bezuglov VV, De Petrocellis L, Di Marzo V (November 2000). "N-acyl-dopamines: novel synthetic CB(1) cannabinoid-receptor ligands and inhibitors of anandamide inactivation with cannabimimetic activity in vitro and in vivo". The Biochemical Journal. 351 Pt 3 (3): 817–24. doi:10.1042/bj3510817. PMC 1221424. PMID 11042139.
- ^ Bisogno T, Ligresti A, Di Marzo V (June 2005). "The endocannabinoid signalling system: biochemical aspects". Pharmacology Biochemistry and Behavior. 81 (2): 224–38. doi:10.1016/j.pbb.2005.01.027. PMID 15935454. S2CID 14186359.
- ^ Ralevic V (July 2003). "Cannabinoid modulation of peripheral autonomic and sensory neurotransmission". European Journal of Pharmacology. 472 (1–2): 1–21. doi:10.1016/S0014-2999(03)01813-2. PMID 12860468.
- ^ Porter AC, Sauer JM, Knierman MD, Becker GW, Berna MJ, Bao J, Nomikos GG, Carter P, Bymaster FP, Leese AB, Felder CC (June 2002). "Characterization of a novel endocannabinoid, virodhamine, with antagonist activity at the CB1 receptor" (PDF). The Journal of Pharmacology and Experimental Therapeutics. 301 (3): 1020–4. doi:10.1124/jpet.301.3.1020. PMID 12023533. S2CID 26156181. Archived from the original (PDF) on 2019-03-03.
- ^ Piñeiro R, Falasca M (April 2012). "Lysophosphatidylinositol signalling: new wine from an old bottle". Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids. 1821 (4): 694–705. doi:10.1016/j.bbalip.2012.01.009. PMID 22285325.
- ^ "What to know about endocannabinoids and the endocannabinoid system". Medical news Today. 27 February 2021. Retrieved 4 August 2021.
- ^ Kano, M.; Ohno-Shosaku, T.; Maejima, T. (2002). "Retrograde signaling at central synapses via endogenous cannabinoids". Molecular Psychiatry. 7 (3): 234–235. doi:10.1038/sj.mp.4000999. PMID 11920149. S2CID 3200861. Retrieved 4 August 2021.
- ^ Fride E, Bregman T, Kirkham TC (April 2005). "Endocannabinoids and food intake: newborn suckling and appetite regulation in adulthood". Experimental Biology and Medicine. 230 (4): 225–34. doi:10.1177/153537020523000401. PMID 15792943. S2CID 25430588.
- ^ Mattes RD, Shaw LM, Engelman K (April 1994). "Effects of cannabinoids (marijuana) on taste intensity and hedonic ratings and salivary flow of adults". Chemical Senses. 19 (2): 125–40. doi:10.1093/chemse/19.2.125. PMID 8055263.
- ^ Yoshida R, Ohkuri T, Jyotaki M, Yasuo T, Horio N, Yasumatsu K, Sanematsu K, Shigemura N, Yamamoto T, Margolskee RF, Ninomiya Y (January 2010). "Endocannabinoids selectively enhance sweet taste". Proceedings of the National Academy of Sciences of the United States of America. 107 (2): 935–9. Bibcode:2010PNAS..107..935Y. doi:10.1073/pnas.0912048107. PMC 2818929. PMID 20080779.
- ^ Vaughan, C. W.; Christie, M. J. (2005). "Retrograde signalling by endocannabinoids". Handbook of Experimental Pharmacology. 168 (168): 367–383. doi:10.1007/3-540-26573-2_12. ISBN 3-540-22565-X. ISSN 0171-2004. PMID 16596781.
- ^ Reynolds, Gretchen (2021-03-10). "Getting to the Bottom of the Runner's High". The New York Times. ISSN 0362-4331. Retrieved 2021-03-16.
- ^ Elsohly MA, Gul W, Wanas AS, Radwan MM (February 2014). "Synthetic cannabinoids: analysis and metabolites". Life Sciences. Special Issue: Emerging Trends in the Abuse of Designer Drugs and Their Catastrophic Health Effects: Update on Chemistry, Pharmacology, Toxicology and Addiction Potential. 97 (1): 78–90. doi:10.1016/j.lfs.2013.12.212. PMID 24412391.
- ^ Lauritsen KJ, Rosenberg H (July 2016). "Comparison of outcome expectancies for synthetic cannabinoids and botanical marijuana". The American Journal of Drug and Alcohol Abuse. 42 (4): 377–84. doi:10.3109/00952990.2015.1135158. PMID 26910181. S2CID 4389339.
- ^ Jump up to: a b "N-(1-amino-3-methyl-1-oxobutan-2-yl)-1-(cyclohexylmethyl)-1H-indazole-3-carboxamide(AB-CHMINACA), N-(1-amino-3-methyl-1-oxobutan-2-yl)-1-pentyl-1H-indazole-3-carboxamide (AB-PINACA)and[1-(5-fluoropentyl)-1H-indazol-3-yl](naphthalen-1-yl)methanone(THJ-2201)" (PDF). Drug and Chemical Evaluation Section, Office of Diversion Control, Drug Enforcement Administration. December 2014. Archived from the original (PDF) on 2018-09-27. Retrieved 2015-01-09. Cite journal requires
|journal=(help) - ^ "More medicinal uses for marijuana". Marijuana.org. October 18, 2005. Archived from the original on 2005-12-21. Retrieved 2014-01-15.
- ^ Rinaldi-Carmona, M.; Barth, F.; Millan, J.; Derocq, J. M.; Casellas, P.; Congy, C.; Oustric, D.; Sarran, M.; Bouaboula, M.; Calandra, B.; Portier, M. (February 1998). "SR 144528, the first potent and selective antagonist of the CB2 cannabinoid receptor". The Journal of Pharmacology and Experimental Therapeutics. 284 (2): 644–650. ISSN 0022-3565. PMID 9454810.
External links[]
 Media related to Cannabinoids at Wikimedia Commons
Media related to Cannabinoids at Wikimedia Commons
- Cannabinoids