Fertaric acid
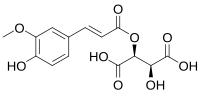 (2S,3S)-stereoisomer
| |
| Names | |
|---|---|
| IUPAC name
2-Hydroxy-3-{[(2E)-3-(4-hydroxy-3methoxyphenyl)prop-2-enoyl]oxy}butandioic acid
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI |
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C14H14O9 | |
| Molar mass | 326.257 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Fertaric acid is a hydroxycinnamic acid found in wine and grapes.[1] It is an ester formed from ferulic acid bound to tartaric acid.
It is a metabolite of caftaric acid after caftaric acid has been fed to rats. Fertaric acid is then found in plasma, kidney, and urine.[2]
References[]
- ^ Branka Mozetič; Irma Tomažič; Andreja Škvarč; Polonca Trebše (2006). "Determination of Polyphenols in White grape Berries cv. Rebula" (PDF). Acta Chim. Slov. 53: 58–64. Archived from the original (PDF) on 2011-08-07.
- ^ Vanzo, A; Cecotti, R; Vrhovsek, U; Torres, AM; Mattivi, F; Passamonti, S (2007). "The fate of trans-caftaric acid administered into the rat stomach". Journal of Agricultural and Food Chemistry. 55 (4): 1604–11. doi:10.1021/jf0626819. PMID 17300159.
Categories:
- O-methylated hydroxycinnamic acids
- Hydroxycinnamic acid esters
- Vinylogous carboxylic acids
- Aromatic compound stubs