From Wikipedia, the free encyclopedia
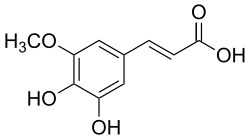
5-Hydroxyferulic acid
|
| Names
|
Preferred IUPAC name
(2E)-3-(3,4-Dihydroxy-5-methoxyphenyl)prop-2-enoic acid |
| Identifiers
|
|
|
|
3D model (JSmol)
|
|
| ChEBI
|
|
| ChemSpider
|
|
| ECHA InfoCard
|
100.230.072 
|
| EC Number
|
|
|
|
|
InChI=1S/C10H10O5/c1-15-8-5-6(2-3-9(12)13)4-7(11)10(8)14/h2-5,11,14H,1H3,(H,12,13)/b3-2+ Key: YFXWTVLDSKSYLW-NSCUHMNNSA-N trans (2E): InChI=1/C10H10O5/c1-15-8-5-6(2-3-9(12)13)4-7(11)10(8)14/h2-5,11,14H,1H3,(H,12,13)/b3-2+ Key: YFXWTVLDSKSYLW-NSCUHMNNBP
|
trans (2E): O=C(O)\C=C\c1cc(O)c(O)c(OC)c1 cis&trans: O=C(O)C=Cc1cc(O)c(O)c(OC)c1 cis: O=C(O)\C=C/c1cc(O)c(O)c(OC)c1
|
| Properties
|
|
|
C10H10O5
|
| Molar mass
|
210.18 g/mol
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). |
| Infobox references
|
|
|
|
Chemical compound
5-Hydroxyferulic acid is a hydroxycinnamic acid.
It is a precursor in the biosynthesis of sinapic acid. Phenylalanine is first converted to cinnamic acid by the action of the enzyme phenylalanine ammonia-lyase (PAL). A series of enzymatic hydroxylations and methylations leads to coumaric acid, caffeic acid, ferulic acid, 5-hydroxyferulic acid and sinapic acid.
Thus 5-hydroxyferulic acid is formed from ferulic acid by the action of the specific enzyme (F5H).
References[]
|
|---|
| Aglycones | | Precursor | |
|---|
Monohydroxycinnamic acids
(Coumaric acids) | |
|---|
| Dihydroxycinnamic acids | |
|---|
| Trihydroxycinnamic acids | |
|---|
| O-methylated forms | |
|---|
| others | |
|---|
|
|---|
| Esters | | glycoside-likes | Esters of
caffeic acid
with cyclitols | |
|---|
| Glycosides | |
|---|
|
|---|
| Tartaric acid esters | |
|---|
Other esters
with caffeic acid | |
|---|
Caffeoyl phenylethanoid
glycoside (CPG) |
- Echinacoside
- , , ,
- , ,
- , , , , , , ,
- Verbascoside (, )
|
|---|
|
|---|
| Oligomeric forms | |
|---|
Conjugates with
coenzyme A (CoA) | |
|---|
Categories:
- O-methylated hydroxycinnamic acids
- Vinylogous carboxylic acids
- Aromatic compound stubs
Hidden categories:
- Chemical articles with multiple compound IDs
- Chemicals using indexlabels
- Chemical articles with multiple CAS registry numbers
- Articles without KEGG source
- Articles without UNII source
- Chembox CAS registry number linked
- Articles with changed CASNo identifier
- Chembox CAS registry number not linked
- ECHA InfoCard ID from Wikidata
- Pages using collapsible list with both background and text-align in titlestyle
- Articles containing unverified chemical infoboxes
- Chembox image size set
- Articles with short description
- Short description matches Wikidata
- All stub articles
