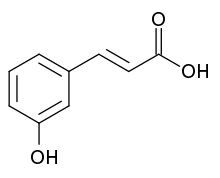
m-Coumaric acid
| |
| Names | |
|---|---|
| Preferred IUPAC name
(2E)-3-(3-Hydroxyphenyl)prop-2-enoic acid | |
| Other names
meta-Coumaric acid
3-Hydroxycinnamic acid | |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.008.742 |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C9H8O3 | |
| Molar mass | 164.16 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
m-Coumaric acid is a hydroxycinnamic acid, an organic compound that is a hydroxy derivative of cinnamic acid.[1] There are three isomers of coumaric acid – o-coumaric acid, m-coumaric acid, and p-coumaric acid – that differ by the position of the hydroxy substitution of the phenyl group.
m-Coumaric acid can be found in vinegar.
References[]
Categories:
- Hydroxycinnamic acids
- Aromatic compound stubs