DEET
| |

| |
| Names | |
|---|---|
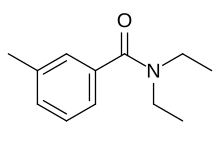
| Preferred IUPAC name
N,N-Diethyl-3-methylbenzamide | |
| Other names
N,N-Diethyl-m-toluamide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.004.682 |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| Properties | |
| C12H17NO | |
| Molar mass | 191.27 g/mol |
| Density | 0.998 g/mL |
| Melting point | −33 °C (−27 °F; 240 K) |
| Boiling point | 288 to 292 °C (550 to 558 °F; 561 to 565 K) |
| Pharmacology | |
| P03BX02 (WHO) QP53GX01 (WHO) | |
| Hazards | |
| Safety data sheet | External MSDS |
| GHS pictograms | 
|
| GHS Signal word | Danger |
GHS hazard statements
|
H302, H315, H319, H402 |
| NFPA 704 (fire diamond) | 
2
1
0 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
N,N-Diethyl-meta-toluamide, also called DEET (/diːt/) or diethyltoluamide, is the most common active ingredient in insect repellents. It is a slightly yellow oil intended to be applied to the skin or to clothing and provides protection against mosquitoes, ticks, fleas, chiggers, leeches and many biting insects.
History[]
DEET was developed in 1944[1] by Samuel Gertler[1] of the United States Department of Agriculture for use by the United States Army,[2] following its experience of jungle warfare during World War II. It was originally tested as a pesticide on farm fields, and entered military use in 1946 and civilian use in 1957. It was used in Vietnam and Southeast Asia.[3]
In its original form, known as "bug juice", the application solution was composed of 75% DEET and 25% ethanol.[4] Later, a new version of the repellent was developed by the U.S. Army and the USDA. This incarnation consisted of DEET and a mixture of polymers that extended its release and reduced its evaporation rate.[4] This extended-release application was registered by the Environmental Protection Agency in 1991.[4]
Preparation[]
A slightly yellow liquid at room temperature, it can be prepared by converting m-toluic acid (3-methylbenzoic acid) to the corresponding acyl chloride using thionyl chloride (SOCl2), and then allowing that product to react with diethylamine:[5][6]
Mechanism and effectiveness[]
DEET was historically believed to work by blocking insect olfactory receptors for 1-octen-3-ol, a volatile substance that is contained in human sweat and breath. The prevailing theory was that DEET effectively "blinds" or "confuses" the insect's senses so that the biting/feeding instinct is not triggered by the chemicals present in the sweat and breath of humans or other animals. DEET does not appear to affect the insect's ability to smell carbon dioxide, as had been suspected earlier.[7][8]
Recent evidence with Anopheles gambiae mosquitoes[9] suggests DEET does not directly inhibit olfactory receptors but instead reduces the volatility of the odorants to which they are attracted.[9][10] By reducing odor volatility, DEET functions to "mask" the ability of volatile odorants on the skin to activate olfactory neurons and attract mosquitoes. A study with adult Anopheles gambiae mosquitoes found no activation of olfactory receptor neurons by DEET.[9] Behavioral tests with Anopheles gambiae mosquitoes indicated that DEET placed five centimetres from a resting mosquito did not repel the mosquito.[9] These data suggest an olfactory function of DEET is to reduce attraction.[9]
In contrast, evidence with Culex quinquefasciatus mosquitoes shows that DEET may serve as a true repellent in that Culex mosquitoes intensely dislike the smell of the chemical.[10] A type of olfactory receptor neuron in special antennal sensilla of mosquitoes that is activated by DEET, as well as other known insect repellents such as eucalyptol, linalool, and thujone, has been identified. Moreover, in a behavioral test, DEET had a strong repellent activity in the absence of body odor attractants such as 1-octen-3-ol, lactic acid, or carbon dioxide. Both female and male mosquitoes showed the same response.[10][11]
The evidence across mosquito species suggests that anthropophilic mosquitoes (such as Anopheles gambiae, Culex quinquefasciatus, and Aedes aegypti) may respond differently to DEET odors. A 2010 study found that Aedes aegypti mosquitoes can rapidly evolve and easily inherit insensitivity to DEET. This insensitivity is provided by an as-yet-unknown dominant gene that can confer resistance even if the trait is inherited from just one parent.[12]
A 2011 structural study (PDB: 3N7H) revealed that DEET binds to Anopheles gambiae odorant binding protein 1 (AgamOBP1) with high shape complementarity, suggesting that AgamOBP1 is a molecular target of DEET and perhaps other repellents.[13] Currently, different studies have focused on the development of new bio-inspired repellents against mosquitoes using as a starting point the DEET structure complexed with AgamOBP1.[14]
A 2013 study suggests that mosquitoes can at least temporarily overcome or adapt to the repellent effect of DEET after an initial exposure, representing a non-genetic behavioral change.[15] This observation, if verified, has significant implications for how repellent effectiveness should be assessed.
A 2019 study indicated that neurons on the tarsi (feet) of Aedes aegypti mosquitoes respond to DEET, and this response repels mosquitoes upon contact.[16] This data indicates DEET functions as a contact repellent.
Concentrations[]
The concentration of DEET in products may range from less than 10 percent to nearly 100 percent. Concentrations of 10 to 30 percent are recommended for infants and children. DEET should not be used on children under 2 months of age.[17]
DEET is often sold and used in spray or lotion in concentrations up to 100%.[18] Consumer Reports found a direct correlation between DEET concentration and hours of protection against insect bites. 100% DEET was found to offer up to 12 hours of protection while several lower concentration DEET formulations (20–34%) offered 3–6 hours of protection.[19] Other research has corroborated the effectiveness of DEET.[20] The Centers for Disease Control and Prevention recommends 30–50% DEET to prevent the spread of pathogens carried by insects.[21]
A 2008 study found that higher concentrations of DEET have an improved ability to repel insects through fabric.[22]
Effects on health[]
As a precaution, manufacturers advise that DEET products should not be used under clothing or on damaged skin, and that preparations be washed off after they are no longer needed or between applications.[23] DEET can act as an irritant;[7] in rare cases, it may cause severe epidermal reactions.[23] Other symptoms that can occur are breathing difficulty, burning eyes, or headaches.[24]
The authors of a 2002 study published in The New England Journal of Medicine wrote, "Despite the substantial attention paid by the lay press every year to the safety of DEET, this repellent has been subjected to more scientific and toxicological scrutiny than any other repellent substance," continuing, "DEET has a remarkable safety profile after 40 years of use and nearly 8 billion human applications," concluding, "When applied with common sense, DEET-based repellents can be expected to provide a safe as well as long-lasting repellent effect."[25]
When DEET is used in combination with insecticides for cockroaches it can strengthen the toxicity of carbamate, an acetylcholinesterase inhibitor. These 1996 findings indicate that DEET has neurological effects on insects in addition to known olfactory effects, and that its toxicity is strengthened in combination with other insecticides.[26]
In the DEET Reregistration Eligibility Decision (RED) in 1998, the United States Environmental Protection Agency (EPA) reported 14 to 46 cases of potential DEET-associated seizures, including four deaths. The EPA states: "... it does appear that some cases are likely related to DEET toxicity," which may underreport the risk as physicians may fail to check for history of DEET use or fail to report cases of seizure subsequent to DEET use.[27]
In 1997, the Pesticide Information Project of Cooperative Extension Offices of Cornell University stated that "Everglades National Park employees having extensive DEET exposure were more likely to have insomnia, mood disturbances and impaired cognitive function than lesser exposed co-workers".[28]
When used as directed, products containing between 10% and 30% DEET have been found by the American Academy of Pediatrics to be safe to use on children, as well as adults, but the Academy recommends that DEET not be used on infants less than two months old.[23]
Citing human health reasons, Health Canada barred the sale of insect repellents for human use that contained more than 30% DEET in a 2002 re-evaluation "based on a human health risk assessment that considered daily application of DEET over a prolonged period of time". The agency recommended that DEET-based products be used on children between the ages of 2 and 12 only if the concentration of DEET is 10% or less and that repellents be applied no more than 3 times a day, children under 2 should not receive more than 1 application of repellent in a day and DEET-based products of any concentration should not be used on infants under 6 months.[29][30] Some experts recommend against applying DEET and sunscreen simultaneously since that would increase DEET penetration; Xiaochen Gu, a professor at the University of Manitoba’s faculty of Pharmacy who led a study about mosquitos, advises that DEET should be applied 30 or more minutes later.[31]
A 2020 study performed by students within the University of Florida's College of Public Health and Health Professions analyzed data from the National Health and Nutrition Examination Survey and identified 1,205 participants who had "DEET metabolic levels recorded at or above detection limits". They analyzed biomarkers related to systemic inflation, immune, liver, and kidney functions, and found no "evidence that DEET exposure has any impact on the biomarkers identified."[32]
Detection in body fluids[]
DEET may be quantitated in blood, plasma, or urine by gas or liquid chromatography-mass spectrometry to confirm a diagnosis of poisoning in hospitalized patients or to provide evidence in a medicolegal death investigation. Blood or plasma DEET concentrations are expected to be in a range of 0.3–3.0 mg/L during the first 8 hours after dermal application in persons using the chemical appropriately, >6 mg/L in intoxicated patients and >100 mg/L in victims of acute intentional oral overdose.[33][34]
Effects on materials[]
DEET is an effective solvent,[7] and may dissolve some watch crystals,[4] plastics, rayon, spandex, other synthetic fabrics, and painted or varnished surfaces including nail polish. It also may act as a plasticizer by remaining inside some formerly hard plastics, leaving them softened and more flexible. DEET is incompatible with rayon, acetate, or dynel clothing.
Effects on the environment[]
Though DEET is not expected to bioaccumulate, it has been found to have a slight toxicity for fresh-water fish such as rainbow trout[35] and tilapia,[36] and it also has been shown to be toxic for some species of freshwater zooplankton.[37] DEET has been detected at low concentrations in water bodies as a result of production and use, such as in the Mississippi River and its tributaries, where a 1991 study detected levels varying from 5 to 201 ng/L.[38]
A 1975 study analyzed the effects of DEET on communities of freshwater organisms native to Chinese waterways and found that compared to other commercial insect repellants, DEET was moderately toxic to aquatic organisms. The most-at-risk organisms were algae colonies which often experienced "significant biomass decline and community composition shift[s]" when exposed to DEET at 500 ng/L.[39]
See also[]
- Beautyberry
- Citronella oil
- DDT, another means of disease vector control
- Icaridin
- Lemon eucalyptus
- Mosquito coil
- p-Menthane-3,8-diol
- Permethrin, a pyrethroid
- SS220
- Anthranilate-based insect repellents
References[]
- ^ Jump up to: a b US 2408389, Gertler S, "N,N-diethylbenzamide as an insect repellent"
- ^ Katz TM, Miller JH, Hebert AA (May 2008). "Insect repellents: historical perspectives and new developments". Journal of the American Academy of Dermatology. 58 (5): 865–71. doi:10.1016/j.jaad.2007.10.005. PMID 18272250. Retrieved 2015-08-16.
- ^ Committee on Gulf War and Health: Literature Review of Pesticides and Solvents (2003). Gulf War and Health: Volume 2. Insecticides and Solvents. Washington, D.C.: National Academies Press. doi:10.17226/10628. ISBN 978-0-309-11389-2.
- ^ Jump up to: a b c d Kitchen LW, Lawrence KL, Coleman RE (June 2009). "The role of the United States military in the development of vector control products, including insect repellents, insecticides, and bed nets". Journal of Vector Ecology. 34 (1): 50–61. doi:10.1111/j.1948-7134.2009.00007.x. PMID 20836805.[permanent dead link]
- ^ Wang BJ (1974). "An interesting and successful organic experiment (CEC)". J. Chem. Educ. 51 (10): 631. doi:10.1021/ed051p631.2.
- ^ Pavia DL (2004). Introduction to organic laboratory techniques (Google Books excerpt). Cengage Learning. pp. 370–376. ISBN 978-0-534-40833-6.
- ^ Jump up to: a b c Petherick A (2008-03-13). "How DEET jams insects' smell sensors". Nature News. doi:10.1038/news.2008.672. Retrieved 2008-03-16.
- ^ Ditzen M, Pellegrino M, Vosshall LB (March 2008). "Insect odorant receptors are molecular targets of the insect repellent DEET". Science. 319 (5871): 1838–42. Bibcode:2008Sci...319.1838D. doi:10.1126/science.1153121. PMID 18339904. S2CID 18499590.
- ^ Jump up to: a b c d e Afify A, Betz JF, Riabinina O, Lahondère C, Potter CJ (November 2019). "Commonly Used Insect Repellents Hide Human Odors from Anopheles Mosquitoes". Current Biology. 29 (21): 3669–3680.e5. doi:10.1016/j.cub.2019.09.007. PMC 6832857. PMID 31630950.
- ^ Jump up to: a b c Syed Z, Leal WS (September 2008). "Mosquitoes smell and avoid the insect repellent DEET". Proceedings of the National Academy of Sciences of the United States of America. 105 (36): 13598–603. doi:10.1073/pnas.0805312105. PMC 2518096. PMID 18711137.
- ^ Fox M, Wiessler D (Aug 18, 2008). "For mosquitoes, DEET just plain stinks". Reuters. Washington. Archived from the original on August 11, 2011. Retrieved August 11, 2011.
- ^ Nagaeva E, Zubarev I, Bengtsson Gonzales C, Forss M, Nikouei K, de Miguel E; et al. (2020). "Heterogeneous somatostatin-expressing neuron population in mouse ventral tegmental area". eLife. 9. doi:10.7554/eLife.59328. PMC 7440918. PMID 32749220.CS1 maint: multiple names: authors list (link)
- ^ Tsitsanou KE, Thireou T, Drakou CE, Koussis K, Keramioti MV, Leonidas DD, et al. (January 2012). "Anopheles gambiae odorant binding protein crystal complex with the synthetic repellent DEET: implications for structure-based design of novel mosquito repellents". Cellular and Molecular Life Sciences. 69 (2): 283–97. doi:10.1007/s00018-011-0745-z. PMID 21671117. S2CID 17986089.
- ^ da Costa KS, Galúcio JM, da Costa CH, Santana AR, Dos Santos Carvalho V, do Nascimento LD, et al. (December 2019). "Exploring the Potentiality of Natural Products from Essential Oils as Inhibitors of Odorant-Binding Proteins: A Structure- and Ligand-Based Virtual Screening Approach To Find Novel Mosquito Repellents". ACS Omega. 4 (27): 22475–22486. doi:10.1021/acsomega.9b03157. PMC 6941369. PMID 31909330.
- ^ Stanczyk NM, Brookfield JF, Field LM, Logan JG (2013). Vontas J (ed.). "Aedes aegypti mosquitoes exhibit decreased repellency by DEET following previous exposure". PLOS ONE (published 20 February 2013). 8 (2): e54438. Bibcode:2013PLoSO...854438S. doi:10.1371/journal.pone.0054438. PMC 3577799. PMID 23437043. Lay summary – BBC news (21 February 2013).
- ^ Dennis EJ, Goldman OV, Vosshall LB (May 2019). "Aedes aegypti Mosquitoes Use Their Legs to Sense DEET on Contact". Current Biology. 29 (9): 1551–1556.e5. doi:10.1016/j.cub.2019.04.004. PMC 6504582. PMID 31031114.
- ^ American Academy of Pediatrics, “Summer Safety Tips,” Dec 2, 2017 https://www.healthychildren.org/English/safety-prevention/at-play/Pages/Summer-Safety-Tips-Staying-Safe-Outdoors.aspx
- ^ Record in the Household Products Database of NLM
- ^ Matsuda BM, Surgeoner GA, Heal JD, Tucker AO, Maciarello MJ (March 1996). "Essential oil analysis and field evaluation of the citrosa plant "Pelargonium citrosum" as a repellent against populations of Aedes mosquitoes". Journal of the American Mosquito Control Association. 12 (1): 69–74. PMID 8723261.
- ^ Williamson D (3 July 2002). "Independent study: DEET products superior for fending off mosquito bites" (Press release). University of North Carolina.
- ^ "Protection against Mosquitoes, Ticks, Fleas and Other Insects and Arthropods". Travelers' Health – Yellow Book. Centers for Disease Control and Prevention. 2009-02-05.
- ^ Ditzen M, Pellegrino M, Vosshall LB (2008). "Insect odorant receptors are molecular targets of the insect repellent DEET". Science. 319 (5871): 1838–42. Bibcode:2008Sci...319.1838D. doi:10.1126/science.1153121. PMID 18339904. S2CID 18499590.CS1 maint: multiple names: authors list (link)
- ^ Jump up to: a b c "Insect Repellent Use and Safety". West Nile Virus. Centers for Disease Control and Prevention. 2007-01-12.
- ^ "Bug spray poisoning". U.S. National Library of Medicine. October 2015. Retrieved 2016-06-25.
- ^ Fradin MS, Day JF (July 2002). "Comparative efficacy of insect repellents against mosquito bites". The New England Journal of Medicine. 347 (1): 13–8. doi:10.1056/NEJMoa011699. PMID 12097535.
- ^ Moss JI (October 1996). "Synergism of toxicity of N,N-diethyl-m-toluamide to German cockroaches (Orthoptera: Blattellidae) by hydrolytic enzyme inhibitors". Journal of Economic Entomology. 89 (5): 1151–5. doi:10.1093/jee/89.5.1151. PMID 17450648.
- ^ "Reregistration Eligibility Decision: DEET" (PDF). U.S. Environmental Protection Agency, Office of Prevention, Pesticides, and Toxic Substances. September 1998. pp. 39–40. Archived from the original (PDF) on October 21, 2012. Retrieved 2012-09-08.
- ^ "DEET". Pesticide Information Profile. EXTOXNET. October 1997. Retrieved 2007-09-26.
- ^ "Insect Repellents". Healthy Living. Health Canada. August 2009. Archived from the original on 2010-04-11. Retrieved 2010-07-09.
- ^ "Re-evaluation Decision Document: Personal insect repellents containing DEET (N,N-diethyl-m-toluamide and related compounds)" (PDF). Consumer Product Safety. Health Canada. 2002-04-15. Retrieved 2010-07-09.
- ^ "How to choose the best bug repellent". Best Health. Reader's Digest Association, Inc. January 2000. Retrieved June 14, 2016.
‘Anything intended for topical use only shouldn’t be going into the body,’ says Xiaochen Gu, a professor at the University of Manitoba’s faculty of pharmacy, who led the study.
- ^ Haleem ZM, Yadav S, Cushion ML, Tanner RJ, Carek PJ, Mainous AG (2020). "Exposure to N,N-Diethyl-Meta-Toluamide Insect Repellent and Human Health Markers: Population Based Estimates from the National Health and Nutrition Examination Survey". Am J Trop Med Hyg. 103 (2): 812–814. doi:10.4269/ajtmh.20-0226. PMC 7410448. PMID 32458781.CS1 maint: multiple names: authors list (link)
- ^ Tenenbein M (September 1987). "Severe toxic reactions and death following the ingestion of diethyltoluamide-containing insect repellents". JAMA. 258 (11): 1509–11. doi:10.1001/jama.258.11.1509. PMID 3625951.
- ^ Baselt RC (2014). Disposition of toxic drugs and chemicals in man, 10th edition. Seal Beach, Ca.: Biomedical Publications. p. 650. ISBN 978-0-9626523-9-4.
- ^ U.S. Environmental Protection Agency. 1980. Office of Pesticides and Toxic Substances. N,N-diethyl-m-toluamide (Deet) Pesticide Registration Standard. December 1980. 83 pp.
- ^ Mathai AT, Pillai KS, Deshmukh PB (1989). "Acute toxicity of deet to a freshwater fish, Tilapia mossambica : Effect on tissue glutathione levels". Journal of Environmental Biology. 10 (2): 87–91. Archived from the original on 2007-11-07.
- ^ Seo J, Lee YG, Kim SD, Cha CJ, Ahn JH, Hur HG (April 2005). "Biodegradation of the insecticide N,N-diethyl-m-toluamide by fungi: identification and toxicity of metabolites". Archives of Environmental Contamination and Toxicology. 48 (3): 323–8. doi:10.1007/s00244-004-0029-9. PMID 15750774. S2CID 31723995.
- ^ Zeiger E, Tice R, Brevard B (1999). "N,N-Diethyl-m-toluamide (DEET) [134-62-3] – Review of Toxicological Literature" (PDF). Archived from the original (PDF) on October 9, 2012.
- ^ Schmoldt A; Benthe HF; Haberland G (1975). "Digitoxin metabolism by rat liver microsomes". Biochem Pharmacol. 24 (17): 1639–41. doi:10.1016/0006-2952(75)90094-5. PMID 10.
Further reading[]
- Fradin MS (June 1998). "Mosquitoes and mosquito repellents: a clinician's guide". Annals of Internal Medicine. 128 (11): 931–40. CiteSeerX 10.1.1.691.2193. doi:10.7326/0003-4819-128-11-199806010-00013. PMID 9634433. S2CID 35046348.
External links[]
| Wikimedia Commons has media related to DEET. |
- DEET General Fact Sheet - National Pesticide Information Center
- DEET Technical Fact Sheet – National Pesticide Information Center
- West Nile Virus Resource Guide – National Pesticide Information Center
- Health Advisory: Tick and Insect Repellents, New York State
- US Centers for Disease Control information on DEET
- US Environmental Protection Agency information on DEET
- Review of scientific literature on DEET (from a RAND Corporation report on Gulf War illnesses)
- Health Canada - Re-evaluation Decision Document: Personal insect repellents containing DEET (N,N-diethyl-m-toluamide and related compounds), 2002
- Household chemicals
- Insect repellents
- Benzamides

