Diamine
General structure of (primary) diamines. The primary amino groups (NH2) are marked blue, R is a divalent organic radical (e.g. a para-phenylene group). |
A diamine is an amine with exactly two amino groups. Diamines are used as monomers to prepare polyamides, polyimides, and polyureas. The term diamine refers mostly to primary diamines, as those are the most reactive.[1]
In terms of quantities produced, 1,6-diaminohexane (a precursor to Nylon 6-6) is most important, followed by ethylenediamine.[2] Vicinal diamines (1,2-diamines) are a structural motif in many biological compounds and are used as ligands in coordination chemistry.[3]
Aliphatic diamines[]
Linear[]
- 1 carbon: methylenediamine (diaminomethane) of theoretical interest only
- 2 carbons: ethylenediamine (1,2-diaminoethane). Related derivatives include the N-alkylated compounds, 1,1-dimethylethylenediamine, 1,2-dimethylethylenediamine, ethambutol, tetrakis(dimethylamino)ethylene, TMEDA.
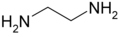
Ethylenediamine
- 3 carbons: 1,3-diaminopropane (propane-1,3-diamine)
- 4 carbons: putrescine (butane-1,4-diamine)
- 5 carbons: cadaverine (pentane-1,5-diamine)
- 6 carbons: hexamethylenediamine (hexane-1,6-diamine), trimethylhexamethylenediamine
Branched[]
Derivatives of ethylenediamine are prominent:
- 1,2-diaminopropane, which is chiral.
- diphenylethylenediamine, two diastereomers, one of which is C2-symmetric.
- 1,2-diaminocyclohexane, two diastereomers, one of which is C2-symmetric.
Cyclic[]
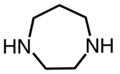
1,4-Diazacycloheptane
Xylylenediamines[]
Xylylenediamines are classified as alkylamines since the amine is not directly attached to an aromatic ring.
- o-xylylenediamine or OXD
- m-xylylenediamine or MXD
- p-xylylenediamine or PXD
Aromatic diamines[]
Three phenylenediamines are known:[4]
- o-phenylenediamine or OPD
- m-phenylenediamine or MPD
- p-phenylenediamine or PPD. 2,5-diaminotoluene is related to PPD but contains a methyl group on the ring.

p-phenylenediamine
Various N-methylated derivatives of the phenylenediamines are known:
- dimethyl-4-phenylenediamine, a reagent.
- N,N'-di-2-butyl-1,4-phenylenediamine, an antioxidant.
Examples with two aromatic rings include derivatives of biphenyl and naphthalene:
- 4,4'-diaminobiphenyl
- 1,8-diaminonaphthalene
References[]
- ^ "Nucleophilicity Trends of Amines". Master Organic Chemistry. 2018-05-07. Retrieved 2019-08-18.
- ^ Karsten Eller, Erhard Henkes, Roland Rossbacher, Hartmut Höke (2005). "Amines, Aliphatic". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a02_001.CS1 maint: uses authors parameter (link)
- ^ Lucet, D., Le Gall, T. and Mioskowski, C. (1998), The Chemistry of Vicinal Diamines. Angew. Chem. Int. Ed., 37: 2580–2627. doi: 10.1002/(SICI)1521-3773(19981016)37:19<2580::AID-ANIE2580>3.0.CO;2-L
- ^ Robert A. Smiley "Phenylene- and Toluenediamines" in Ullmann's Encyclopedia of Industrial Chemistry 2002, Wiley-VCH, Weinheim. doi:10.1002/14356007.a19_405
External links[]
- Diamines at the US National Library of Medicine Medical Subject Headings (MeSH)
- Synthesis of diamines
- Diamines
- Monomers

