Dynemicin A

| |
| Names | |
|---|---|
| IUPAC name
(1S,4R,4aR,14S,14aS,18Z)-6,8,11-trihydroxy-3-methoxy-1-methyl-7,12-dioxo-1,4,7,12,13,14-hexahydro-4a,14a-epoxy-4,14-hex[3]ene[1,5]diynonaphtho[2,3-c]phenanthridine-2-carboxylic acid
| |
| Other names
(2R,4S,5S,8R,11Z,15S)-21,24,28-trihydroxy-7-methoxy-5-methyl-19,26-dioxo-3-oxa-16-azaheptacyclo[15.12.0.02,4.02,8.04,15.018,27.020,25]nonacosa-1(29),6,11,17,20,22,24,27-octaen-9,13-diyne-6-carboxylic acid
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C30H19NO9 | |
| Molar mass | 537.473 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Dynemicin A is an anti-cancer enediyne drug. It displays properties which illustrate promise for cancer treatments, but still requires further research.
History and background[]
Dynemicin A was first isolated from the soil in the Gujarat State of India. It was discovered to be the natural product of the indigenous bacteria . The natural product displays a bright purple color due to the anthraquinone chromophore structure within it. Initially, this compound was isolated for its aesthetic properties as a dye until further research demonstrated its anti-cancer properties. Shortly after the compound's discovery, the Bristol-Myers Pharmaceutical Company elucidated the structure in Japan using X-ray diffraction studies of triacetyldynemicin A; a closely related compound.
Synthesis[]
The first reported chemical synthesis of dynemicin was accomplished by Myers and coworkers.[1]
Biosynthesis[]
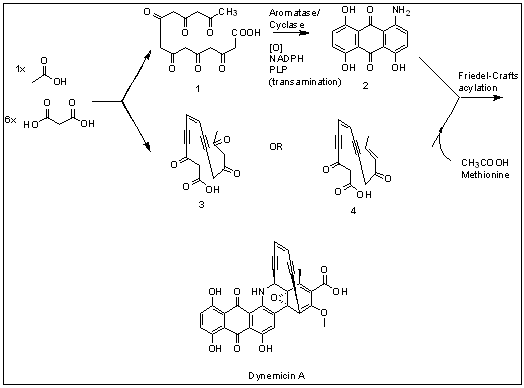
Dynemicin A is an antitumor natural product isolated from Micromonospora chersina which causes DNA strand cleavage. Iwasaki et al. first studied the biosynthetic pathway of Dynemicin A by 13C NMR labeling experiments.[2] Dynemicin A is thought to be biosynthesized separately from two different heptaketide chains originated from seven head-to-tail coupled acetate units, which is then connected to form Dynemicin A. Initially, precursors such as 3 and 4 were proposed to derive from the oleate/crepenynate pathway, as initially put forth for NSC Chrom A biosynthesis.[3] However, recent work by Thorson and coworkers revealed the biosynthesis of the dynemicin enediyne core to be catalyzed by an enediyne polyketide synthase (PKSE) similar to that employed in calicheamicin biosynthesis.[4][5]
Mechanism of action[]
Dynemicin A is specific for B-DNA, and functions by intercalating into the minor groove of the double helix. For intercalation to occur, the separation between strands, which is usually 3-4 angstroms, needs to be widened to 7-8 angstroms to allow enough space for the ligand to bind. Given this, the DNA must be strained to accommodate the Dynemicin A, resulting in an induced fit-like process. Once intercalated within the DNA, the epoxide is activated in one of two ways. First, if NADPH or a thiol reduces the molecule the Bergman re-cyclization of the enediyne proceeds. Second, if a nucleophilic mechanism is utilized then the retro-Bergman re-cyclization of the enediyne is used. The final products of these two mechanisms are outlined below. When the re-cyclization occurs, the conformational changes and chemical reactions taking place result in an irreversible double stranded cleavage of the DNA, leading to cell death. During in vitro studies, the molecule showed an increased affinity for the specific 10 base pair sequence CTACTACTTG. However, in vivo studies have yet to confirm this phenomenon. Professor Martin Semmelhack of Princeton University was the first person to propose the NADPH reduction pathway.
Pharmacological properties[]
The pharmacological properties of this drug have not yet been fully explored but currently suggest that it may be a more potent anti-cancer agent than other chemotherapeutic drugs. The bacterium is believed to use dynemicin A as an antibacterial agent to help it survive in its niche in the environment. Dynemicin A, as a drug, specifically targets B-DNA and is most effective in rapidly dividing cells. The broad spectrum of the drug prevents current use because it creates unwanted damage in normal healthy tissues. In vivo studies in mice and rats suggest that the treatment is most effective in leukemia, breast, and lung cancers. Synthetic alternatives which are more specific to cancer cells and leave healthy tissues unharmed are being researched. Other animal models are available, but have proven ineffective and therefore have no human trials currently underway. The enediyne property of this drug relates to another antibiotic known as neocarzinostatin which is approved for clinical use. As with dynemicin A, neocarzinostatin also interacts with DNA.
References[]
This article has an unclear citation style. (May 2011) |
- ^ Myers, AG; Fraley, ME; Tom, NJ; Cohen, SB; Madar, DJ (January 1995). "Synthesis of (+)-dynemicin A and analogs of wide structural variability: establishment of the absolute configuration of natural dynemicin A." Chemistry & Biology. 2 (1): 33–43. doi:10.1016/1074-5521(95)90078-0. PMID 9383401.
- ^ Tokiwa, Y.; Miyoshi-Saitoh, M.; Kobayashi, H.; Sunaga, R.; Konishi, M.; Oki, T.; Iwasaki, S (1992). "Biosynthesis of dynemicin A, a 3-ene-1,5-diyne antitumor antibiotic". J. Am. Chem. Soc. 114 (11): 4107–4110. doi:10.1021/ja00037a011.
- ^ Hensens, O. D.; Giner, J.; Goldberk, I. H (1989). "Biosynthesis of NCS Chrom A, the chromophore of the antitumor antibiotic neocarzinostatin". J. Am. Chem. Soc. 111 (9): 3295–3299. doi:10.1021/ja00191a028.
- ^ Liu, W; Ahlert, J; Gao, Q; Wendt-Pienkowski, E; Shen, B; Thorson, JS (14 October 2003). "Rapid PCR amplification of minimal enediyne polyketide synthase cassettes leads to a predictive familial classification model". Proceedings of the National Academy of Sciences of the United States of America. 100 (21): 11959–63. Bibcode:2003PNAS..10011959L. doi:10.1073/pnas.2034291100. PMC 218695. PMID 14528002.
- ^ Gao, Q; Thorson, JS (May 2008). "The biosynthetic genes encoding for the production of the dynemicin enediyne core in Micromonospora chersina ATCC53710". FEMS Microbiology Letters. 282 (1): 105–14. doi:10.1111/j.1574-6968.2008.01112.x. PMC 5591436. PMID 18328078.
Notes[]
- ElSohly, Adel. "Dynemicin A: Molecule in Review." Columbia University. 12 June 2009.
- Liew, C. W.; Scharff, A.; Kotaka, M.; Kong, R.; Sun, H.; Qureshi, I.; Bricogne, G.; Liang, Z.; Lescar, J. (2010). "Induced-fit upon Ligand Binding Revealed by Crystal Structures of the Hot-dog Fold Thioesterase in Dynemicin Biosynthesis". J. Mol. Biol. 404 (2): 291–306. doi:10.1016/j.jmb.2010.09.041. PMID 20888341.
- Nicolaou, K. C., S. A. Snyder, A. G. Meyers, and S. J. Danishefsky. "Dynemicin A." Classics in Total Synthesis II: More Targets, Strategies, Methods. Weinheim: Wiley-VCH, 2003. 75–107.
- Schulz-Aellen, Marie-Françoise. "Cancer Drugs." Aging and Human Longevity. Boston: Birkhäuser, 1997. 203-04
- Silverman, Richard B. "Dynemicin A." The Organic Chemistry of Drug Design and Drug Action. Amsterdam: Elsevier Academic, 2004. 381-85
- Tuttle, Tell; Kraka, Elfi; Cremer, Dieter (2005). "Docking, Triggering, and Biological Activity of Dynemicin A in DNA: A Computational Study". Journal of the American Chemical Society. 127 (26): 9469–484. doi:10.1021/ja046251f. PMID 15984874.
- Tuttle, Tell; Kraka, Elfi; Theil, Walter; Cremer, Dieter (2007). "A QM/MM Study of the Bergman Reaction of Dynemicin A in the Minor Groove of DNA". J Phys Chem. 111 (28): 8321–328. doi:10.1021/jp072373t. PMID 17585802.
External links[]
- https://web.archive.org/web/20100626231055/http://www.columbia.edu/cu/chemistry/groups/synth-lit/MIR2009/2009_06_12-AElsohly-Dynemycin.pdf
- http://www.pdb.org/pdb/explore/pubmedArticle.do?structureId=2XFL#
- https://books.google.com/books?id=PE587tLs3w0C&pg=PA76&lpg=PA76&dq=Bristol-Myers+structure+dynemicin+a&source=bl&ots=X-l9kgIwfu&sig=H6RCeET9W75_KSupYJhxx3gYRsc&hl=en&ei=_CG6TdWJL8rz0gHYusRt&sa=X&oi=book_result&ct=result&resnum=5&ved=0CDgQ6AEwBA#v=onepage&q=Bristol-Myers%20structure%20dynemicin%20a&f=false
- https://books.google.com/books?id=7l6IOF2t3vIC&pg=PA203&lpg=PA203&dq=where+does+dynemicin+a+come+from&source=bl&ots=fcczlI2BvW&sig=mUWUqTwG283RgGvAxUARhqMJNcw&hl=en&ei=GKe0TabeNouO0QGSwPSfCQ&sa=X&oi=book_result&ct=result&resnum=1&ved=0CBYQ6AEwAA#v=onepage&q=dynemycin&f=false
- https://web.archive.org/web/20120405090957/http://smu.edu/catco/pdf/jacs_127_9469.pdf
- https://web.archive.org/web/20121001181112/http://smu.edu/catco/pdf/JPC-B_111_8321.pdf
- http://www.britannica.com/EBchecked/media/81/Examples-of-anthraquinone-pigments
- http://www.chem.strath.ac.uk/people/academic/tell_tuttle/research/qmmm
- Cancer research
- Enediynes
- Angucyclines
- Carboxylic acids
- Epoxides
- Triols
- Nitrogen heterocycles
- Heterocyclic compounds with 7 or more rings
- Ten-membered rings