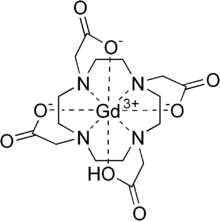
Gadoteric acid
 | |
| Clinical data | |
|---|---|
| Trade names | Artirem, Dotarem, Clariscan, others[1] |
| Other names | DOTA-Gd, gadoterate meglumine |
| AHFS/Drugs.com | International Drug Names |
| License data | |
| Routes of administration | Intravenous |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C16H25GdN4O8 |
| Molar mass | 558.65 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Gadoteric acid, sold under the brand name Dotarem among others, is a macrocycle-structured gadolinium-based MRI contrast agent (GBCA). It consists of the organic acid DOTA as a chelating agent, and gadolinium (Gd3+), and is used in form of the meglumine salt (gadoterate meglumine).[3][4] The paramagnetic property of gadoteric acid reduces the T1 relaxation time (and to some extent the T2 and T2* relaxation times) in MRI, which is the source of its clinical utility. Because it has magnetic properties, gadoteric acid develops a magnetic moment when put under a magnetic field, which increases the signal intensity (brightness) of tissues during MRI imaging.[6]
Usage[]
It is used for imaging of blood vessels and inflamed or diseased tissue where the blood vessels become 'leaky'. It is often used when viewing intracranial lesions with abnormal vascularity or abnormalities in the blood–brain barrier. Gadoteric acid is used for MRI imaging of the brain, spine, and associated tissues for adult and pediatric (2 years of age or older) patients. The meglumine salt it takes the form of crosses the blood brain barrier of tissue with abnormal vasculature, highlighting the affected area with MRI. Gadoterate does not cross the intact blood-brain barrier, so it does not affect or enhance normal brain tissue in imaging.[6] Dotarem is administered through an intravenous bolus injection, either manually or through a power injection.[3][4]
Adverse effects[]
It is retained in the brain at a measurable level after an injection at standard dose (0.1 mmol/kg).[7] In vitro studies found it neurotoxic, less so than linears agents.[8]
Drugs with gadolinium-based contrasting agents can cause nephrogenic systemic fibrosis (NSF, or gadolinium-induced fibrosis) for those with impaired elimination of the drug. Those most at risk for NSF include patients with chronic or severe kidney disease and acute kidney injury.[3][4][9]
The most common adverse effects (>0.2%) in clinical studies were nausea, headache, injection site pain, injection site coldness, and burning sensation.
Bioequivalence[]
A 2020 study found Clariscan was retained more in the cerebrum, cerebellum, kidney and liver of rats than those injected Dotarem,[10] its original drug. Although the authors didn't provide a possible explanation, differences in the chelation process of gadolinium ions (Guerbet's process being patented) or quality assurance could be causes of increased retention in vivo.
Availability[]
The drug, under the brand name Dotarem, was brought to market by Guerbet.[11] It was launched on French market in 1989 and was FDA-approved in USA in March 2013.[11] As of 2013, gadoteric acid was approved in around 70 countries.[12][13] Dotarem is the seventh FDA-approved GBCA for use in central nervous system MRI.[citation needed]
In 2019, GE Healthcare launched gadoteric acid medication (as gadoterate meglumine) under the brand name Clariscan.[4][14]
References[]
- ^ "Gadoteric Acid International". Drugs.com. 6 January 2021. Retrieved 21 January 2021.
- ^ "Dotagraf 0.5 mmol/ml solution for injection - Summary of Product Characteristics (SmPC)". (emc). Retrieved 29 August 2021.
- ^ a b c d "Dotarem- gadoterate meglumine injection". DailyMed. Retrieved 29 August 2021.
- ^ a b c d e "Clariscan- gadoterate meglumine injection, solution". DailyMed. Retrieved 29 August 2021.
- ^ https://www.ema.europa.eu/documents/psusa/gadoteric-acid-intra-articular-formulation-list-nationally-authorised-medicinal-products-psusa/00001505/202004_en.pdf
- ^ a b DrugBank, ed. (22 August 2016). "Gadoteric acid". DrugBank.
- ^ Stanescu, A. Luana; Shaw, Dennis W.; Murata, Nozomu; Murata, Kiyoko (March 2020). "Brain tissue gadolinium retention in pediatric patients after contrast-enhanced magnetic resonance exams: pathological confirmation". Pediatric Radiology. 50 (3): 388–396. doi:10.1007/s00247-019-04535-w. ISSN 1432-1998. PMID 31989188. Retrieved 24 August 2021.
- ^ Bower, Danielle V.; Richter, Johannes K.; von Tengg-Kobligk, Hendrik; Heverhagen, Johannes T. (August 2019). "Gadolinium-Based MRI Contrast Agents Induce Mitochondrial Toxicity and Cell Death in Human Neurons, and Toxicity Increases With Reduced Kinetic Stability of the Agent". Investigative Radiology. 54 (8): 453–463. doi:10.1097/RLI.0000000000000567. ISSN 1536-0210. PMID 31265439. Retrieved 21 August 2021.
- ^ Todd DJ, Kay J (2016). "Gadolinium-Induced Fibrosis". Annual Review of Medicine. 67: 273–91. doi:10.1146/annurev-med-063014-124936. PMID 26768242.
- ^ Bussi, Simona; Coppo, Alessandra; Celeste, Roberto; Fanizzi, Antonello; Fringuello Mingo, Alberto; Ferraris, Andrea; Botteron, Catherine; Kirchin, Miles A.; Tedoldi, Fabio; Maisano, Federico (4 February 2020). "Macrocyclic MR contrast agents: evaluation of multiple-organ gadolinium retention in healthy rats". Insights into Imaging. 11. doi:10.1186/s13244-019-0824-5. ISSN 1869-4101. PMC 7000570. PMID 32020385.
- ^ a b Hollmer M (6 January 2014). "Dotarem: A safe(r) gadolinium-based contrast imaging agent". FierceBiotech.
- ^ "Gadoteric Acid international brands". Drugs.com. Retrieved 7 March 2017.
- ^ Guerbet LLC (14 February 2013). "Advisory Committee Briefing Document for NDA 204-781". FDA.
- ^ "Clariscan 0.5 mmol/ ml solution for injection" (PDF). Archived from the original (PDF) on 1 March 2017. Retrieved 28 February 2017.
Further reading[]
- Lancelot E (November 2016). "Revisiting the Pharmacokinetic Profiles of Gadolinium-Based Contrast Agents: Differences in Long-Term Biodistribution and Excretion". Invest Radiol. 51 (11): 691–700. doi:10.1097/RLI.0000000000000280. PMID 27175546. S2CID 38922357.
External links[]
- "Gadoteric acid". Drug Information Portal. U.S. National Library of Medicine.
- "Gadoterate meglumine". Drug Information Portal. U.S. National Library of Medicine.
- Organogadolinium compounds
- MRI contrast agents