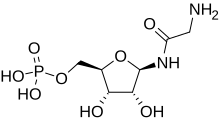
Glycineamide ribonucleotide
| |
| Names | |
|---|---|
| Preferred IUPAC name
[(2R,3S,4R,5R)-5-(2-Aminoacetamido)-3,4-dihydroxyoxolan-2-yl]methyl dihydrogen phosphate | |
| Other names
Glycineamide ribotide,
GAR | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| MeSH | Glycineamide+ribonucleotide |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C7H15N2O8P | |
| Molar mass | 286.177 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Glycineamide ribonucleotide (or GAR) is an intermediate in de novo purine biosynthesis.
It is formed from phosphoribosylamine by the enzyme phosphoribosylamine—glycine ligase. In the next step of purine biosynthesis the enzyme phosphoribosylglycinamide formyltransferase acts on GAR to form phosphoribosyl-N-formylglycineamide (FGAR).
GAR formation is stimulated by luteinizing hormone (LH) and chorionic gonadotropin (HCG) via activation of Glc-6-P-dehydrogenase (EC 1.1.1.49)[1][2]
Synonyms[]
Several names are associated with GAR:
- 5′-p-Ribosylglycinamide
- 5′-p-Ribosylglycineamide
- 5′-Phosphoribosylglycinamide
- 5′-Phosphoribosylglycineamide
- Glycineamide ribotide
- Glycinamide ribonucleotide
- Glycineamide ribonucleotide
- N1-(5-Phospho-D-ribosyl)glycinamide
- N-Glycyl-5-O-phosphono-D-ribofuranosylamine
- N1-(5-phospho-D-ribosyl)glycinamide
References[]
- ^ "SMPDB: Glycineamideribotide". August 24, 2015.
- ^ "Human Metabolome Database: Glycineamideribotide". August 24, 2015.
Categories:
- Organic compound stubs
- Nucleotides