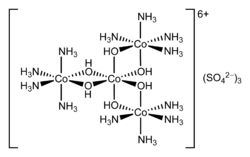
Hexol
| |

| |
| Names | |
|---|---|
| IUPAC name
Tris[tetrammine-μ-dihydroxocobalt(III)]cobalt (III) ion
| |
| Identifiers | |
3D model (JSmol)
|
|
| |
| |
| Properties | |
| Co4H42N12O18S3 | |
| Molar mass | 830.31 g·mol−1 |
| Sparingly soluble in water [1] | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Hexol is the name for various salts of the coordination complex {[Co(NH3)4(OH)2]3Co}6+. These salts are of historical significance as the first synthetic non-carbon-containing chiral compounds.[2] The sulfate salt has the formula {[Co(NH3)4(OH)2]3Co}(SO4)3(H2O)x.[3]
Preparation and optical resolution[]
Salts of hexol were first described by Jørgensen,[4] although it was Werner who recognized its structure.[5] The salt is prepared by heating [Co(NH3)4(H2O)2]3+ with dilute base such as ammonia followed by precipitation of the sulfate salt:[1]
- 4 [Co(NH3)4(H2O)2]3+ → {[Co(NH3)4(OH)2]3Co}6+ + 4 NH4+ + 2 H+ + 2 H2O
Depending on the conditions one obtains the 9-hydrate, the 6-hydrate, and the 4-hydrate. These salts exists as dark brownish-violet or black tabular crystals. The salts have low solubility in water. When treated with concentrated hydrochloric acid, hexol converts to cis-diaquotetramminecobalt(III) sulfate. In boiling dilute sulfuric acid, hexol degrades with evolution of oxygen and nitrogen.[1]
Optical resolution[]
In a historic set of experiments, {[Co(NH3)4(OH)2]3Co}6+ was resolved by fractional crystallisation of its D-(+)-bromocamphorsulfonate salt.[5] A more efficient resolution involves the bis(tartrato)diantimonate(III) anion. The hexol hexacation has a high specific rotation of 2640°.[6] It belongs to the D3 point group.
"Second hexol"[]
Werner also described a second achiral hexol (a minor byproduct from the production of Fremy's salt) that he incorrectly identified as a linear tetramer. The second hexol is hexanuclear (contains six cobalt centres in each ion), not tetranuclear.[7] Its point group is C2h, and its formula is [Co
6(NH
3)
14(OH)
8O
2]6+
, whereas that of hexol is [Co
4(NH
3)
12(OH)
6]6+
.
References[]
- ^ a b c Kauffman, George B.; Pinnell, Robert P. (1960). "Tris[tetrammine-μ-dihydroxo-cobalt(III)]cobalt(III) Sulfate 4-Hydrate". Tris[Tetrammine-μ-Dihydroxo-Cobalt(III)] Cobalt(III) Sulfate 4-Hydrate. Inorganic Syntheses. 6. pp. 176–179. doi:10.1002/9780470132371.ch56. ISBN 9780470132371.
- ^ Miessler, G. L. and Tarr, D. A. Inorganic Chemistry, 3rd ed., Pearson/Prentice Hall publisher, ISBN 0-13-035471-6.
- ^ Ernst, Karl-Heinz; Berke, Heinz (2011). "Optical Activity and Alfred Werner's Coordination Chemistry". Chirality. 23 (3): 187–189. doi:10.1002/chir.20912. PMID 20928897.
- ^ Jørgensen, S. M. (1898). "Zur Konstitution der Kobalt-, Chrom- und Rhodiumbasen". Zeitschrift für Anorganische Chemie. 16: 184–197. doi:10.1002/zaac.18980160116.
- ^ a b Werner, A. (1907). "Über mehrkernige Metallammoniake" [Poly-nucleated Metal-amines]. Ber. Dtsch. Chem. Ges. (in German). 40 (2): 2103–2125. doi:10.1002/cber.190704002126.
- ^ Yasui, Takaji; Ama, Tomoharu; Kauffman, George B. (1992). "Resolution of the Dodecaamminehexa-μ-Hydroxo-Tetracobalt(III) Ion". Inorganic Syntheses. Inorganic Syntheses. 29. pp. 169–174. doi:10.1002/9780470132609.ch41. ISBN 9780470132609.
- ^ Jackson, W. Gregory; McKeon, Josephine A.; Zehnder, Margareta; Neuberger, Markus; Fallab, Silvio (2004). "The rediscovery of Alfred Werner's second hexol". Chemical Communications (20): 2322–2323. doi:10.1039/B408277J. PMID 15490001.
External links[]
- Hexol Molecule of the Month September 1997 Website
- National Pollutant Inventory – Cobalt fact sheet
- Cobalt compounds
- Stereochemistry
- Coordination compounds
