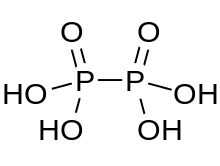
Hypophosphoric acid
| |
| Names | |
|---|---|
| IUPAC name
Hypodiphosphoric acid
| |
| Other names
Diphosphoric acid
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| H4P2O6 | |
| Molar mass | 161.98 g/mol |
| Appearance | White solid (dihydrate) |
| Melting point | 54 °C (129 °F; 327 K) |
| Conjugate base | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Hypophosphoric acid is a mineral acid with the formula H4P2O6, with phosphorus in a formal oxidation state of +4. In the solid state it is present as the dihydrate, H4P2O6·2H2O. In hypophosphoric acid the phosphorus atoms are identical and joined directly with a P−P bond. Isohypophosphoric acid is a structural isomer of hypophosphoric acid in which one phosphorus has a hydrogen directedly bonded to it and that phosphorus atom is linked to the other one by an oxygen bridge to give a phosphorous acid/phosphoric acid mixed anhydride. The two phosphorus atoms are in the +3 and +5 oxidation states, respectively.
Preparation and reactions[]
Hypophosphoric acid can be prepared by the reaction of red phosphorus with sodium chlorite at room temperature.[1]
- 2 P + 2 NaClO2 + 2 H2O → Na2H2P2O6 + 2 HCl
A mixture of hypophosphoric acid, phosphorous acid (H3PO3) and phosphoric acid (H3PO4) is produced when white phosphorus oxidises in air when partially immersed in water.[1]
The tetrasodium salt Na4P2O6·10H2O crystallises at pH 10 and the disodium salt, Na2H2PO6·6H2O at pH 5.2.[2] The disodium salt can be passed through an ion exchange column to form the acid dihydrate, H4P2O6·2H2O.[1]
The anhydrous acid can be formed by vacuum dehydration over P4O10 or by the reaction of H2S on lead hypophosphate, Pb2P2O6.[2]
Hypophosphoric acid is tetraprotic with dissociation constants pKa1 = 2.2, pKa2 = 2.8, pKa3 = 7.3 and pKa4 = 10.0.[2]
On standing the anhydrous acid undergoes rearrangement and disproportionation to form a mixture of isohypophosphoric acid, HPO(OH)-O-PO2(OH); pyrophosphoric acid H2P2O7 and pyrophosphorous acid.[2]
Hypophosphoric acid is unstable in hot hydrochloric acid, in 4 M HCl it hydrolyses to give H3PO3 + H3PO4.[2]
Structure[]
Hypophosphorus acid contains oxonium ions and is best formulated [H3O+]2 [H2P2O6]2−. The acid is isostructural with the diammonium salt which contains the [HOPO2PO2OH]2− anion with a P−P bond length of 219 pm.[1]
The HOPO2PO2OH2− anion in Na2H2P2O6·6H2O has a symmetric, staggered ethane-like structure with a P−P bond of length 219 pm. Each phosphorus atom has two P−O bonds with length 151 pm, and a P−OH bond length of 159 pm.[3]
Hypophosphate salts[]
Many hypophosphate salts are known, for example, K4P2O6·8H2O, Ca2P2O6·2H2O, K3HP2O6·3H2O, K2H2P2O6·2H2O, KH3P2O6.
On standing in air, hypophosphates tend to oxidise to pyrophosphates containing the P
2O4−
7 ion where P has a formal oxidation state of +5. Hypophosphates are stable to alkali hydroxides. In fused sodium hydroxide they convert rapidly to the orthophosphate containing PO3−
4.[1]
Polyhypophosphates[]
Polyhypophosphates are known containing linear anions, for example Na5P3O8 containing O(PO2)3O5− with a P−P−P chain and Na6P4O10·2H2O containing O(PO2)4O6−, with a P−P−P−P chain. The cyclic anion (PO
2)6−
6, (hypohexametaphosphate[4]) where each phosphorus atom has an oxidation state of +3 is formed when a suspension of red phosphorus in KOH is oxidised with bromine.[1]
See also[]
- Dithionic acid, the sulfur equivalent.
References[]
- ^ a b c d e f Phosphorus: Chemistry, Biochemistry and Technology, Sixth Edition, 2013, D.E.C. Corbridge, CRC Pres, Taylor Francis Group, ISBN 978-1-4398-4088-7
- ^ a b c d e Greenwood, Norman N.; Earnshaw, Alan (1984). Chemistry of the Elements. Oxford: Pergamon Press. pp. 515–516. ISBN 978-0-08-022057-4.
- ^ Collin, R. L.; Willis, M. (1971). "The crystal structure of disodium dihydrogen hypophosphate hexahydrate (Na2H2P2O6·6H2O) and disodium dihydrogen pyrophosphate hexahydrate (Na2H2P2O7·6H2O)". Acta Crystallographica Section B. 27 (2): 291–302. doi:10.1107/S0567740871002127. ISSN 0567-7408.
- ^ Holleman, Arnold Frederik; Wiberg, Egon (2001), Wiberg, Nils (ed.), Inorganic Chemistry, translated by Eagleson, Mary; Brewer, William, San Diego/Berlin: Academic Press/De Gruyter, p. 715, ISBN 0-12-352651-5
- Mineral acids
- Phosphorus oxoacids