Icosahedral twins



This article needs additional citations for verification. (August 2016) |
An icosahedral twin is a nanostructure appearing for atomic clusters. These clusters are twenty-faced, made of ten interlinked dual-tetrahedron (bowtie) crystals, typically joined along triangular (e.g. cubic-(111)) faces having three-fold symmetry. One can think of their formation as a kind of atom-scale self-assembly.
A variety of nanostructures (e.g. condensing argon, metal atoms, and virus capsids) assume icosahedral form on size scales where surface forces eclipse those from the bulk. A twinned form of these nanostructures is sometimes found to occur e.g. in face-centered-cubic (FCC) metal-atom clusters.[1] This may occur when the building blocks beneath each of the 20 facets of an initially icosahedral cluster (which cannot fill space without defects) "make the case" (as the surface-to-volume ratio of these facets decreases with size) for conversion to a translationally symmetric (e.g. defect-free face-centered-cubic) crystalline form[2]
Causes[]
When interatom bonding does not have strong directional preferences, it is not unusual for atoms to gravitate toward a kissing number of 12 nearest neighbors. The three most symmetric ways to do this are by icosahedral clustering, or by crystalline face-centered-cubic (cuboctahedral) and/or hexagonal (tri-orthobicupolar) close packing.
Icosahedral arrangements, perhaps because of their slightly smaller surface area, may be preferred for small clusters e.g. noble gas and metal atoms in condensed phases (both liquid[3] and solid). However, the Achilles heel for icosahedral clustering about a single point is that it cannot fill space over large distances in a way that is translationally ordered.
Hence bulk atoms (i.e. sufficiently large clusters) generally revert to one of the crystalline close-packing configurations instead. In other words, when icosahedral clusters get sufficiently large, the bulk-atom vote wins out over the surface-atom vote, and the atoms beneath each of the 20 facets adopt a face-centered-cubic pyramidal arrangement with tetrahedral (111) facets. Thus icosahedral twins are born, with a certain amount of strain along the interfacial (111) planes.
Ubiquity[]
Icosahedral twinning has been seen in face-centered-cubic metal nanoparticles that have nucleated: (i) by evaporation onto surfaces, (ii) out of solution, and (iii) by reduction in a polymer matrix.
Quasicrystals are un-twinned structures with long range rotational but not translational periodicity, that some initially tried to explain away as icosahedral twinning.[4] Quasi-crystals let non-space-filling coordination persist to larger size scales. However, they generally form only when the compositional makeup (e.g. of two dissimilar metals like Ti and Mn) serves as an antagonist to formation of one of the more common close-packed space-filling but twinned crystalline forms.
Application[]
Face-centered-cubic noble metal atomic clusters are important nano-catalysts for chemical reactions. One example of this is the platinum used in automobile catalytic converters. Icosahedral twinning makes it possible to cover the entire surface of a nanoparticle with {111} facets, in cases where those particular atomic-facets show favorable catalytic activity.
Detection[]
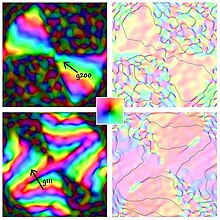
Electron diffraction and high-resolution transmission electron microscopy (TEM) imaging are two methods for identifying the icosahedral-twin structure of individual clusters. Digital dark field analysis of lattice-fringe images shows promise for recognition of icosahedral twinning from most of the randomly oriented clusters in a microscope-image field of view.[5]
See also[]
- Crystal twinning
- Dark field microscopy
- Icosahedron
- Nanomaterial based catalyst
- Nanotechnology
- Quasicrystals
- Self-assembly of nanoparticles
Footnotes[]
- ^ H. Hofmeister (2004) "Fivefold twinned nanoparticles" in Encyclopedia of Nanoscience and Nanotechnology (ed. H. S. Nalwa, Amer. Sci. Publ., Stevenson Ranch CA) vol. 3, pp. 431-452 ISBN 1-58883-059-4 pdf.
- ^ K. F. Kelton of Washington University in St. Louis, USA and A. L. Greer of University of Cambridge, UK (2010) Nucleation in Condensed Matter: Applications in Materials and Biology (Elsevier Science & Technology, Amsterdam) link.
- ^ Kelton, K. F.; Lee, G. W.; Gangopadhyay, A. K.; Hyers, R. W.; Rathz, T. J.; et al. (2003-05-15). "First X-Ray Scattering Studies on Electrostatically Levitated Metallic Liquids: Demonstrated Influence of Local Icosahedral Order on the Nucleation Barrier". Physical Review Letters. American Physical Society (APS). 90 (19): 195504. doi:10.1103/physrevlett.90.195504. ISSN 0031-9007.
- ^ Pauling, Linus (1987-01-26). "So-called icosahedral and decagonal quasicrystals are twins of an 820-atom cubic crystal". Physical Review Letters. American Physical Society (APS). 58 (4): 365–368. doi:10.1103/physrevlett.58.365. ISSN 0031-9007.
- ^ Fraundorf, P.; Bishop, C. (2013). "Efficient Lattice-Image Detection of Icosahedral Twins". Microscopy and Microanalysis. Cambridge University Press (CUP). 19 (S2): 1804–1805. doi:10.1017/s143192761301101x. ISSN 1431-9276.
- Nanoparticles