Immunological memory
Immunological memory is the ability of the immune system to quickly and specifically recognize an antigen that the body has previously encountered and initiate a corresponding immune response. Generally these are secondary, tertiary and other subsequent immune responses to the same antigen. Immunological memory is responsible for the adaptive component of the immune system, special T and B cells — the so-called memory T and B cells. Immunological memory is the basis of vaccination.[1][2] Emerging resources show support for the innate immune system's participation in immune memory responses in invertebrates as well as vertebrates.[3][4]
Development of immunological memory[]
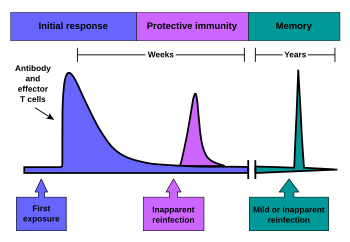
Immunological memory occurs after a primary immune response against the antigen. Immunological memory is thus created by each individual, after a previous initial exposure, to a potentially dangerous agent. The course of secondary immune response is similar to primary immune response. After the memory B cell recognizes the antigen it presents the peptide: MHC I complex to nearby effector T cells. That leads to activation of these cells and rapid proliferation of cells. After the primary immune response has disappeared, the effector cells of the immune response are eliminated.[5] However, there remain antibodies previously created in the body that represent the humoral component of immunological memory and comprise an important defensive mechanism in subsequent infections. In addition to the formed antibodies in the body there remains a small number of memory T and B cells that make up the cellular component of the immunological memory. They stay in blood circulation in a resting state and at the subsequent encounter with the same antigen these cells are able to respond immediately and eliminate the antigen. Memory cells have a long life and last up to several decades in the body.[6][2]
Immunity to chickenpox, measles, and some other diseases lasts a lifetime. Immunity to many diseases eventually wears off. The immune system's response to a few diseases, such as dengue, counterproductively makes the next infection worse (antibody-dependent enhancement).[7]
As of 2019, researchers are still trying to find out why some vaccines produce life-long immunity, while the effectiveness of other vaccines drops to zero in less than 30 years (for mumps) or less than six months (for H3N2 influenza).[8]
Evolution of immune memory[]
The evolutionary invention of memory T and B cells is widespread; however, the conditions required to develop this costly adaptation are specific. First, in order to evolve immune memory the initial molecular machinery cost must be high and will demand losses in other host characteristics. Second, middling or long lived organisms have higher chance of evolving such apparatus. The cost of this adaption increases if the host has a middling lifespan as the immune memory must be effective earlier in life.[9]
Furthermore, research models show that the environment plays an essential role in the diversity of memory cells in a population. Comparing the influence of multiple infections to a specific disease as opposed to disease diversity of an environment provide evidence that memory cell pools accrue diversity based on the number of individual pathogens exposed, even at the cost of efficiency when encountering more common pathogens. Individuals living in isolated environments such as islands will have a less diverse population of memory cells, but present with sturdier immune responses. This indicates that the environment plays a large role in the evolution of memory cell populations.[10]
Previously acquired immune memory can be depleted by measles in unvaccinated children, leaving them at risk of infection by other pathogens in the years after infection.[11]
Memory B cells[]
Memory B cells are plasma cells that are able to produce antibodies for a long time. Unlike the naive B cells involved in the primary immune response the memory B cell response is slightly different. The memory B cell has already undergone clonal expansion, differentiation and affinity maturation, so it is able to divide multiple times faster and produce antibodies with much higher affinity (especially IgG).[1] In contrast, the naive plasma cell is fully differentiated and cannot be further stimulated by antigen to divide or increase antibody production. Memory B cell activity in secondary lymphatic organs is highest during the first 2 weeks after infection. Subsequently, after 2 to 4 weeks its response declines. After the germinal center reaction the memory plasma cells are located in the bone marrow which is the main site of antibody production within the immunological memory.[12]
Memory T cells[]
Memory T cells can be both CD4+ and CD8+. These memory T cells do not require further antigen stimulation to proliferate; therefore, they do not need a signal via MHC.[13] Memory T cells can be divided into two functionally distinct groups based on the expression of the CCR7 chemokine receptor. This chemokine indicates the direction of migration into secondary lymphatic organs. Those memory T cells that do not express CCR7 (these are CCR7-) have receptors to migrate to the site of inflammation in the tissue and represent an immediate effector cell population. These cells were named memory effector T cells (TEM). After repeated stimulation they produce large amounts of IFN-γ, IL-4 and IL-5. In contrast, CCR7 + memory T cells lack proinflammatory and cytotoxic function but have receptors for lymph node migration. These cells were named central memory T cells (TCM). They effectively stimulate dendritic cells, and after repeated stimulation they are able to differentiate in CCR7- effector memory T cells. Both populations of these memory cells originate from naive T cells and remain in the body for several years after initial immunization.[14]
Innate immune memory[]
The innate immune system is also involved in aspects of immune memory, despite not having the ability to manufacture antibodies like the adaptive immune system. In a process known as Trained Immunity, many invertebrates such as species of fresh water snails, copepod crustaceans, and tapeworms have been observed activating innate immune memory to instigate a more efficient immune response to specific antigens without an adaptive branch of the immune system.[3] Mice without functional T and B cells were able to survive the administration of a lethal dose of Candida albicans when exposed previously to a much smaller amount, showing that vertebrates also retain this ability.[4] While PAMPs can both prime the innate immune system's cells to take on certain characteristics adaptive characteristics, overexertion of the signals involved can become harmful to the host.[3] Presence of DAMPs can lead to modulation of inflammation by the innate immune system. Trained immunity is thought to be driven by a number of factors including epigenetics, metabolic rate, and transcriptional reprogramming.[3][4] A key part of this response is the limitation of the immune response in order to avoid tissue damage on secondary infections which is referred to as innate immune tolerance.[3][4]
See also[]
References[]
- ^ a b Murphy, Kenneth; Weaver, Casey (2017). Janeway's Immunology (9th ed.). New York & London: Garland Science. pp. 473–475. ISBN 9780815345510.
- ^ a b Hammarlund, Erika, et al. (2003). "Duration of antiviral immunity after smallpox vaccination." Nature medicine 9.9, 1131.
- ^ a b c d e Crișan, Tania O.; Netea, Mihai G.; Joosten, Leo A. B. (April 2016). "Innate immune memory: Implications for host responses to damage-associated molecular patterns". European Journal of Immunology. 46 (4): 817–828. doi:10.1002/eji.201545497. ISSN 0014-2980. PMID 26970440.
- ^ a b c d Gourbal, Benjamin; Pinaud, Silvain; Beckers, Gerold J. M.; Van Der Meer, Jos W. M.; Conrath, Uwe; Netea, Mihai G. (2018-04-17). "Innate immune memory: An evolutionary perspective". Immunological Reviews. 283 (1): 21–40. doi:10.1111/imr.12647. ISSN 0105-2896. PMID 29664574. S2CID 4891922.
- ^ Sprent, Jonathan, and Susan R. Webb. "Intrathymic and extrathymic clonal deletion of T cells." Current opinion in immunology 7.2 (1995): 196-205.
- ^ Crotty, Shane, et al. "Cutting edge: long-term B cell memory in humans after smallpox vaccination." The Journal of Immunology 171.10 (2003): 4969-4973.
- ^ Ed Yong. "Immunology Is Where Intuition Goes to Die". 2020. quote: "Immunity lasts a lifetime for some diseases—chickenpox, measles—but eventually wears off for many others." quote: "For some diseases, like dengue, an antibody response to one infection can counterintuitively make the next infection more severe."
- ^ Jon Cohen. "How long do vaccines last?". 2019.
- ^ Best, Alex; Hoyle, Andy (2013-06-06). "The evolution of costly acquired immune memory". Ecology and Evolution. 3 (7): 2223–2232. doi:10.1002/ece3.611. ISSN 2045-7758. PMC 3728959. PMID 23919164.
- ^ Graw, Frederik; Magnus, Carsten; Regoes, Roland R (2010). "Theoretical analysis of the evolution of immune memory". BMC Evolutionary Biology. 10 (1): 380. doi:10.1186/1471-2148-10-380. ISSN 1471-2148. PMC 3018457. PMID 21143840.
- ^ Mina MJ, Kula T, Leng Y, Li M, Vries RD, Knip M, et al. (2019-11-01). "Measles virus infection diminishes preexisting antibodies that offer protection from other pathogens". Science. 366 (6465): 599–606. Bibcode:2019Sci...366..599M. doi:10.1126/science.aay6485. hdl:10138/307628. ISSN 0036-8075. PMID 31672891. S2CID 207815213.
- ^ Slifka, Mark K., Mehrdad Matloubian, and Rafi Ahmed (1995). "Bone marrow is a major site of long-term antibody production after acute viral infection." Journal of Virology, 69(3), 1895–1902.
- ^ Kassiotis, George, et al. "Impairment of immunological memory in the absence of MHC despite survival of memory T cells." Nature immunology 3.3 (2002): 244.
- ^ Sallusto, Federica, et al. "Two subsets of memory T lymphocytes with distinct homing potentials and effector functions." Nature 401.6754 (1999): 708.
- Immune system