Iridin
| |
| Names | |
|---|---|
| IUPAC name
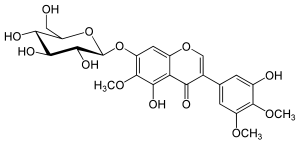
7-(β-D-Glucopyranosyloxy)-3′,5-dihydroxy-4′,5′,6-trimethoxyisoflavone
| |
| Preferred IUPAC name
5-Hydroxy-3-(3-hydroxy-4,5-dimethoxyphenyl)-6-methoxy-7-{[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}-4H-1-benzopyran-4-one | |
| Other names
Irisin[1]
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C24H26O13 | |
| Molar mass | 522.45 g/mol |
| Melting point | 208 °C (406 °F; 481 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Iridin is an isoflavone, a type of flavonoid. It is the 7-glucoside of irigenin and can be isolated from several species of irises like orris root, Iris florentina[2] or Iris versicolor, also commonly known as the larger blue flag. It can also be found in Iris kemaonensis.[3][4]
The compound is toxic and these plants have been mentioned as causing poisoning in humans and animals.[5]
References[]
- ^ Iridin on chestofbooks.com
- ^ Iridin on drugs.com
- ^ Agarwal, V.K.; Thappa, R.K.; Agarwal, S.G.; Mehraa, M.S.; Dhar, K.L. (1984). "Isoflavones of two Iris species". Phytochemistry. 23 (11): 2703–2704. doi:10.1016/S0031-9422(00)84141-2.
- ^ J. B. Harborne The Flavonoids: Advances in Research since 1980, p. 133, at Google Books
- ^ Yellow Iris on cbif.gc.ca Archived 2011-06-10 at the Wayback Machine
Categories:
- O-methylated isoflavones
- Isoflavone glucosides
- Hydroxymethyl compounds
- Aromatic compound stubs