Licochalcone A
| |
| Names | |
|---|---|
| Preferred IUPAC name
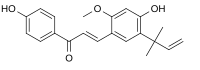
(2E)-3-[4-Hydroxy-2-methoxy-5-(2-methylbut-3-en-2-yl)phenyl]-1-(4-hydroxyphenyl)prop-2-en-1-one | |
| Other names
Licochalcone a
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.163.544 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C21H22O4 | |
| Molar mass | 338.403 g·mol−1 |
| Hazards | |
| Safety data sheet (SDS) | [1] |
| GHS labelling: | |
 [1] [1]
| |
Signal word
|
Warning |
| H302, H312, H332[1] | |
| P261, P264, P280[1] | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Licochalcone A is a chalconoid, a type of natural phenol. It can be isolated from the root of Glycyrrhiza glabra[2] (liquorice) or Glycyrrhiza inflata.[3] It shows antimalarial, anticancer, antibacterial and antiviral (specifically against influenza neuraminidase) properties in vitro.[2][3][4][5]
References[]
- ^ a b c "Licochalcone A Safety Data Sheet" (PDF). Cayman Chemicals.
- ^ a b Fu, Y.; Hsieh, T. C.; Guo, J.; Kunicki, J.; Lee, M. Y. W. T.; Darzynkiewicz, Z.; Wu, J. M. (2004). "Licochalcone-A, a novel flavonoid isolated from licorice root (Glycyrrhiza glabra), causes G2 and late-G1 arrests in androgen-independent PC-3 prostate cancer cells". Biochemical and Biophysical Research Communications. 322 (1): 263–270. doi:10.1016/j.bbrc.2004.07.094. PMID 15313200.
- ^ a b Friis-Møller, A.; Chen, M.; Fuursted, K.; Christensen, S. R. B. G.; Kharazmi, A. (2002). "In Vitro Antimycobacterial and Antilegionella Activity of Licochalcone a from Chinese Licorice Roots". Planta Medica. 68 (5): 416–419. doi:10.1055/s-2002-32087. PMID 12058317.
- ^ Chen, M.; Theander, T. G.; Christensen, S. B.; Hviid, L.; Zhai, L.; Kharazmi, A. (1994). "Licochalcone A, a new antimalarial agent, inhibits in vitro growth of the human malaria parasite Plasmodium falciparum and protects mice from P. Yoelii infection". Antimicrobial Agents and Chemotherapy. 38 (7): 1470–1475. doi:10.1128/aac.38.7.1470. PMC 284578. PMID 7979274.
- ^ Dao, TT; Nguyen, PH; Lee, HS; Kim, E; Park, J; Lim, SI; Oh, WK (January 2011). "Chalcones as novel influenza A (H1N1) neuraminidase inhibitors from Glycyrrhiza inflata". Bioorganic & Medicinal Chemistry Letters. 21 (1): 294–8. doi:10.1016/j.bmcl.2010.11.016. PMID 21123068.
Categories:
- Chalconoids
- Aromatic compound stubs