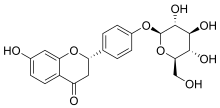
Liquiritin
This article provides insufficient context for those unfamiliar with the subject. (October 2014) |
| |
| Names | |
|---|---|
| IUPAC name
(2S)-4′-(β-D-Glucopyranosyloxy)-7-hydroxyflavan-4-one
| |
| Preferred IUPAC name
(2S)-7-Hydroxy-4-(4-{[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}phenyl)-2,3-dihydro-4H-1-benzopyran-4-one | |
| Other names
Liquiritoside
Liquiritigenin-4'-O-glucoside | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C21H22O9 | |
| Molar mass | 418.398 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Liquiritin is the 4'-O-glucoside of the flavanone liquiritigenin. Liquiritin is one of flavone compounds derived from licorice.[1]
De novo biosynthesis of liquiritin in Saccharomyces cerevisiae using endogenous yeast metabolites as precursors and cofactors, provides a possibility for the economical and sustainable production and application of licorice flavonoids through synthetic biology.[2]
References[]
- ^ Cong JX, Wang SY, Wu XH, Yu P (2012). "Optimization of Separation Conditions of Liquiritin in Preparative Liquid Chromatography". Advanced Materials Research. 550–553: 1647–1652. doi:10.4028/www.scientific.net/AMR.550-553.1647. S2CID 97214921.
- ^ Yin Y, Li Y, Jiang D, Zhang X, Gao W, Liu C (April 2020). "De novo biosynthesis of liquiritin in Saccharomyces cerevisiae". Acta Pharmaceutica Sinica B. 10 (4): 711–721. doi:10.1016/j.apsb.2019.07.005. PMC 7161706. PMID 32322472.
Further reading[]
- Wang J, Wang D, Yu J, Liu C, Li L, Zhang Y (April 2014). "Isolation of liquiritigenin-4'-apiosyl-glucoside and liquiritin from the root of Glycyrrhiza uralensis by high-performance centrifugal partition chromatography". Journal of Chromatographic Science. 52 (4): 310–4. doi:10.1093/chromsci/bmt029. PMID 23552847.
- Cong, Jing Xiang; Wang, Shao Yan; Gao, Hong (2012). "Separation of Liquiritin by Two-Dimensional Liquid Chromatography". Advanced Materials Research. 455–456: 1232–1238. doi:10.4028/www.scientific.net/AMR.455-456.1232. ISSN 1662-8985. S2CID 135570958.
- Cong J, Lin B (March 2007). "Separation of Liquiritin by simulated moving bed chromatography". Journal of Chromatography A. 1145 (1–2): 190–4. doi:10.1016/j.chroma.2007.01.088. PMID 17289063.
- Ni H, Xu M, Xie K, Fei Y, Deng H, He Q, Wang T, Liu S, Zhu J, Xu L, Yao M (2020). "Liquiritin Alleviates Pain Through Inhibiting CXCL1/CXCR2 Signaling Pathway in Bone Cancer Pain Rat". Frontiers in Pharmacology. 11: 436. doi:10.3389/fphar.2020.00436. PMC 7193085. PMID 32390832.
External links[]
 Media related to Liquiritin at Wikimedia Commons
Media related to Liquiritin at Wikimedia Commons
Categories:
- Flavonoid glucosides
- Flavanone glycosides
- Aromatic compound stubs