Mars Oxygen ISRU Experiment
 Mars Oxygen In-Situ Resource Utilization Experiment (MOXIE) | |
| Operator | NASA |
|---|---|
| Manufacturer | NASA/Caltech Jet Propulsion Laboratory |
| Instrument type | ISRU (in situ resource utilization) experimental technology |
| Function | Oxygen production |
| Website | mars |
| Properties | |
| Mass | 15 kg (33 lb) |
| Dimensions | 24 × 24 × 31 cm |
| Power consumption | 300 W |
| Host spacecraft | |
| Spacecraft | Perseverance |
| Launch date | July 30, 2020 |
| Rocket | Atlas V 541 |
| Launch site | Cape Canaveral SLC-41 |
The Mars Oxygen In-Situ Resource Utilization Experiment (MOXIE)[1] is a technology demonstration on the NASA Mars 2020 rover Perseverance investigating the production of oxygen on Mars.[2] On April 20, 2021, MOXIE produced oxygen from carbon dioxide in the Martian atmosphere by using solid oxide electrolysis. This was the first experimental extraction of a natural resource from another planet for human use.[1][3] The technology may be scaled up for use in a human mission to the planet to provide breathable oxygen, oxidizer, and propellant; water may also be produced by combining the produced oxygen with hydrogen.[4]
The experiment was a collaboration between the Massachusetts Institute of Technology, the Haystack Observatory, the NASA/Caltech Jet Propulsion Laboratory, and other institutions.
Objective[]
MOXIE's objective is to produce oxygen of at least 98% purity at a rate of 6–10 grams per hour (0.21–0.35 oz/h) and to do this at least ten times, so the device can be tested in a range of times of the day, including at night, and in most environmental conditions, including during a dust storm.[1]
Development[]



MOXIE builds upon an earlier experiment, the Mars In-situ propellant production Precursor (MIP), which was designed and built to fly on the Mars Surveyor 2001 Lander mission.[5] MIP was intended to demonstrate In-Situ Propellant Production (ISPP) on a laboratory scale using electrolysis of carbon dioxide to produce oxygen.[6] The MIP flight demonstration was postponed when the Mars Surveyor 2001 lander mission was cancelled after the Mars Polar Lander mission failed.[7] [8]
The Principal Investigator (PI) of MOXIE is Michael Hecht from the Haystack Observatory at Massachusetts Institute of Technology (MIT).[9] The deputy PI is former NASA astronaut Jeffrey Hoffman from the Department of Aeronautics and Astronautics at MIT. The project manager is Jeff Mellstrom from the NASA/Caltech Jet Propulsion Laboratory (JPL). Along with MIT and JPL, major contributors are OxEon Energy (previously Ceramatec, Inc.) and Air Squared. Other contributors include Imperial College London, Space Exploration Instruments LLC, Destiny Space Systems LLC, the Niels Bohr Institute at the University of Copenhagen, Arizona State University, and the Technical University of Denmark.[10][11]
Principle[]
MOXIE acquires, compresses, and heats Martian atmospheric gases using a HEPA filter, scroll compressor, and heaters alongside insulation,[1] then splits the carbon dioxide (CO
2) molecules into oxygen (O) and carbon monoxide (CO) using solid oxide electrolysis, where the O atoms combine to form gaseous oxygen (O
2).[12]
The conversion process requires a temperature of approximately 800 °C (1,470 °F).[4] A solid oxide electrolysis cell works on the principle that, at elevated temperatures,[12] certain ceramic oxides, such as yttria-stabilized zirconia (YSZ) and doped ceria, become oxide ion (O2–) conductors. A thin nonporous disk of YSZ (solid electrolyte) is sandwiched between two porous electrodes. CO
2 diffuses through the porous electrode (cathode) and reaches the vicinity of the electrode-electrolyte boundary. Through a combination of thermal dissociation and electrocatalysis, an oxygen atom is liberated from the CO
2 molecule and picks up two electrons from the cathode to become an oxide ion (O2–). Via oxygen ion vacancies in the crystal lattice of the electrolyte, the oxygen ion is transported to the electrolyte–anode interface due to the applied DC potential. At this interface, the oxygen ion transfers its charge to the anode, combines with another oxygen atom to form oxygen (O
2), and diffuses out of the anode.[1]
The net reaction is thus 2 CO
2 2 CO + O
2. Inert gases such as nitrogen gas (N
2) and argon (Ar) are not separated from the feed, but returned to the atmosphere with the carbon monoxide (CO) and unused CO
2.[1]
Mars experiment[]
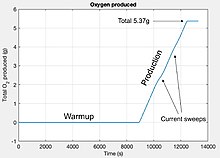
Oxygen production was first achieved on April 20, 2021 in Jezero Crater, producing 5.37 grams (0.189 oz) of oxygen, equivalent to what an astronaut on Mars would need to breathe for roughly 10 minutes.[13] MOXIE is designed to safely generate up to 10 g/h (0.35 oz/h) of oxygen,[14][4] with theoretical production limited to 12 grams per hour (0.42 oz/h) of oxygen due to the limited capacity of the 4 ampere flight power supply.[1] The oxygen produced is analyzed, and then released back into the atmosphere.[15]
MOXIE is planned to isolate oxygen a further nine more times over the course of approximately two Earth years, or one Martian year, in three stages; the first stage will further investigate the oxygen production, the second to test the instrument in a variety of times of day, seasons, and atmospheric conditions, and the third to produce oxygen at different temperatures, and alter the mode of operation to investigate differences in production.[4]
On April 21, 2021, Jim Reuter, the Associate Administrator for STMD explained that the experiment was functioning with results having many uses, stating: "This is a critical first step at converting carbon dioxide to oxygen on Mars. MOXIE has more work to do, but the results from this technology demonstration are full of promise as we move toward our goal of one day seeing humans on Mars. Oxygen isn’t just the stuff we breathe. Rocket propellant depends on oxygen, and future explorers will depend on producing propellant on Mars to make the trip home."[13]
Implications[]
NASA states that if MOXIE worked efficiently, they could land an approximately 200-times larger, MOXIE-based instrument on the planet, along with a power plant capable of generating 25–30 kilowatts (34–40 hp).[1] Over the course of approximately one Earth year, this system would produce oxygen at a rate of at least 2 kilograms per hour (4.4 lb/h)[1] in support of a human mission sometime in the 2030s.[16][17] The stored oxygen could be used for life support, but the primary need is for an oxidizer for a Mars ascent vehicle.[18][19] It is projected for example, in a mission of four astronauts on Martian surface for a year, only about 1 metric ton of oxygen would be used for life support for the entire year, compared to about 25 metric tons of oxygen for propulsion off the surface of Mars for the return mission.[13] The CO, a byproduct of the reaction, may be collected and used as a low-grade fuel[20] or reacted with water to form methane (CH
4) for use as a primary fuel.[21][22] As an alternative use, an oxygen generation system could fill a small oxygen tank to support a sample return mission.[23] The oxygen could also be combined with hydrogen to form water.[4]
Technical Specifications[]
Data from NASA (MARS 2020 Mission Perseverance Rover),[9] Ceramatec and OxEon Energy,[24] NASA Jet Propulsion Laboratory.[25]
• Main Job: To produce oxygen from the Martian carbon-dioxide atmosphere.
• Location: Inside the rover (front, right side)
• Mass: 17.1 kilograms
• Weight: 37.7 pounds on Earth, 14.14 pounds on Mars
• Power: 300 watts
• Volume: 9.4 x 9.4 x 12.2 inches (23.9 x 23.9 x 30.9 centimeters)
• Oxygen Production Rate: Up to 10 grams per hour (At least 0.022 pounds per hour)
• Operation Time: Approximately one hour of oxygen (O2) production per experiment. which will be scheduled intermittently over the duration of the mission.[9]
MOXIE: Operational Design Drive (SOXE):
• Gas Flow: Internally manifolded for O2 Purity & dP
• Feed: DRY CO2 in a range of 30-80 g/hr
• Product: 99.6% Pure O2, internal manifolding
• Structural: Robust to survive Launch, EDL Shock and Vibe, Compression Load Requirements
• Power: Highly constrained
• Mass: 1 kg max
• Volume: Rigidly constrained
• Operation: 20+ 120 minute cycles
• Heating Ramps: 90 minutes (~515°C/hour) from ambient(potentially -40°C) to 800°C.
• Heat Application: Heaters on endplates only[24]
MOXIE: Materials Design Drivers:
• Interconnects (IC): Powder metallurgy (CFY, Plansee)
• Seals: Glass Seals
• Current Busbars: Brazed rod / welded wire
• Feed Manifolds: Inlet tube/internal manifold O2 purity
• Anode Electrode: Perovskite
• Cathode Electrode: Modifiedproprietary Cermet
• Electrolyte: Scandia-Stabilized Zirconia (ScSZ)[24]
MOXIE: Cell Design:
• Number of Cells: 10 Cells (Arranged in two stacks of 5 cells each)
• Oxygen Production: 10 grams per hour (>1g/hr. per cell)[25]
• Each Cell Consists of:
- Electrolyte (Yttria-Stabilized Zirconia YSZ)
- Cathode
- Anode
• Connecting Cells:
- High chromium alloy (matched CTE to ceramic electrolyte)
- Approximately 100mm x 50mm x 2mm
- Contains manifolding for gas streams[25]
MOXIE: Gas Delivery System (Scroll Compressor):
• Scroll Pump Compression Rate: Up to approximately 1 bar
• Scroll Pump RPM: Low-speed (2000-4000 RPM)
• Performance: Inlet Gas: 83g/hr, P= 7 Torr, T= 20°C, Pin= 120 W, Mass: ~2kg[25]
MOXIE: Targets:
• Operational Cycles: The primary mission requirements call for the capability to operate a total of 20 cycles:[26]
– 10 cycles preflight
– 10+ cycles on Mars
• Qualification and Verification Testing: It involves 60 full operational cycles for proof of extensibility, which is three times the number of cycles planned for the primary mission.[26]
• Oxygen Purity: 99.6%+ at end of life
• Temperature Capability: Capable to operate at -65°C proof temperature
• Compression, Shock, and Vibe Requirements:
- Withstand 8 kN compressive force
- Withstand (PF) + 3dB levels for flight shock and vibe requirements[24]
References[]
- ^ Jump up to: a b c d e f g h i Hecht, M.; Hoffman, J.; Rapp, D.; McClean, J.; SooHoo, J.; Schaefer, R.; Aboobaker, A.; Mellstrom, J.; Hartvigsen, J.; Meyen, F.; Hinterman, E. (2021-01-06). "Mars Oxygen ISRU Experiment (MOXIE)". Space Science Reviews. 217 (1): 9. Bibcode:2021SSRv..217....9H. doi:10.1007/s11214-020-00782-8. ISSN 1572-9672. S2CID 106398698.
- ^ Beutel, Allard (2015-04-15). "NASA Announces Mars 2020 Rover Payload to Explore the Red Planet". NASA. Archived from the original on 2021-02-19. Retrieved 2021-02-25.
- ^ "Nasa device extracts breathable oxygen from thin Martian air". The Irish Times. Archived from the original on 2021-04-22. Retrieved 2021-04-22.
- ^ Jump up to: a b c d e Potter, Sean (2021-04-21). "NASA's Perseverance Mars Rover Extracts First Oxygen from Red Planet". NASA. Archived from the original on 2021-04-22. Retrieved 2021-04-22.
- ^ Kaplan, David; Baird, R.; Flynn, Howard; Ratliff, James; Baraona, Cosmo; Jenkins, Phillip; Landis, Geoffrey; Scheiman, David; Johnson, Kenneth; Karlmann, Paul (2000). "The 2001 Mars In-situ-propellant-production Precursor (MIP) Flight Demonstration - Project objectives and qualification test results". Space 2000 Conference and Exposition. doi:10.2514/6.2000-5145.
- ^ Flavell, Waryn (15 March 2021). "Making Oxygen on Mars is No Match for This Johnson Team". NASA Johnson Space Center Features. Archived from the original on 22 April 2021. Retrieved 22 April 2021.
- ^ "nasa". www.history.nasa.gov. Archived from the original on 2019-07-14. Retrieved 2021-04-22.
- ^ Colombano, Silvano (23 September 2003). "Robosphere: Self-Sustaining Robotic Ecologies as Precursors to Human Planetary Exploration". AIAA Space 2003 Conference & Exposition. doi:10.2514/6.2003-6278. Retrieved 25 July 2021.
- ^ Jump up to: a b c mars.nasa.gov. "Mars Oxygen In-Situ Resource Utilization Experiment (MOXIE)". mars.nasa.gov. Archived from the original on 2021-02-27. Retrieved 2021-02-25.
- ^ "NASA TechPort – Mars OXygen ISRU Experiment Project". NASA TechPort. National Aeronautics and Space Administration. Archived from the original on 17 October 2020. Retrieved 19 November 2015.
- ^ Brix, Lise (26 April 2015). "Scientists are trying to brew oxygen on Mars". Science Nordic. Archived from the original on 2015-04-30. Retrieved 2015-05-15.
- ^ Jump up to: a b "Game Changing Development The Mars Oxygen ISRU Experiment (MOXIE)" (PDF). National Aeronautics and Space Administration. Archived (PDF) from the original on 3 December 2020. Retrieved 22 April 2021.
- ^ Jump up to: a b c Potter, Sean (2021-04-21). "NASA's Perseverance Mars Rover Extracts First Oxygen from Red Planet". NASA. Archived from the original on 2021-04-22. Retrieved 2021-04-23.
- ^ "Aboard NASA's Perseverance rover, MOXIE creates oxygen on Mars". MIT News | Massachusetts Institute of Technology. Archived from the original on 2021-04-21. Retrieved 2021-04-22.
- ^ Cody, Sara (29 July 2020). "With Perseverance and a little MOXIE, MIT is going to Mars". MIT News. Massachusetts Institute of Technology. Retrieved 20 May 2021.
- ^ The Mars Oxygen ISRU Experiment (MOXIE) Archived 2016-12-22 at the Wayback Machine PDF. Presentation: MARS 2020 Mission and Instruments". November 6, 2014.
- ^ Maxey, Kyle (August 5, 2014). "Can Oxygen Be Produced on Mars? MOXIE Will Find Out". Engineering.com. Archived from the original on 2014-11-06. Retrieved 2014-11-05.
- ^ Thomson, Iain (31 July 2014). "Mars rover 2020: Oxygen generation and 6 more amazing experiments". The Register. Archived from the original on 2014-11-06. Retrieved 2014-11-05.
- ^ Living off the Land in the Final Frontier Archived November 4, 2014, at the Wayback Machine. NASA, 4th November 2014.
- ^ Landis, Geoffrey A.; Linne, Diane L. (September–October 2001). "Mars Rocket Vehicle Using In Situ Propellants". Journal of Spacecraft and Rockets. 38 (5): 730–735. Bibcode:2001JSpRo..38..730L. doi:10.2514/2.3739.
- ^ Wall, Mike (August 1, 2014). "Oxygen-Generating Mars Rover to Bring Colonization Closer". Space.com. Archived from the original on 2014-11-04. Retrieved 2014-11-05.
- ^ "Ceramic Oxygen Generator for Carbon Dioxide Electrolysis Systems | SBIR.gov". www.sbir.gov. Archived from the original on 2014-11-06. Retrieved 2014-11-06.
- ^ Landis, Geoffrey A.; Oleson, Steven R.; Packard, Thomas W.; Linne, Diane L.; Woytach, Jeffrey M.; Martini, Michael C.; Fittje, James E.; Gyekenyesi, John Z.; Colozza, Anthony J.; Fincannon, James; Bury, Kristen M.; Dominguez, Hector; Jones, Robert; Smith, David; Vento, Daniel (9–13 January 2017). Design Study of a Mars Ascent Vehicle for Sample Return Using In-Situ Generated Propellant. 10th Symposium on Space Resource Utilization. Grapevine, Texas. doi:10.2514/6.2017-0424.
- ^ Jump up to: a b c d J. Hartvigsen, S. Elangovan, J. Elwell, D. Larsen, L. Clark, E. Mitchel, B. MilletCeramatec, Inc/OxEonEnergy Development and Flight Qualification of a Solid Oxide CO2 Electrolysis Stack for the Mars2020 MOXIE Project https://www.ice2017.net
- ^ Jump up to: a b c d Aboobaker, Asad (18 September 2017). "MOXIE: Generating Oxygen On Mars" (PDF). NASA Jet Propulsion Laboratory. California Institute of Technology. Retrieved 5 May 2021.
- ^ Jump up to: a b Hartvigsen, Joseph; Elangovan, S.; Frost, Lyman (8 July 2018). "MOXIE Development Driven Prospects for ISRU and Atmosphere Revitalization" (PDF). 48th International Conference on Environmental Systems.
External links[]
| Wikimedia Commons has media related to Mars Oxygen ISRU Experiment (MOXIE). |
- Colonization of Mars
- Electrochemical cells
- Mars 2020 instruments
- Oxygen
- Space science experiments
- Technology demonstrations

