Mecoprop
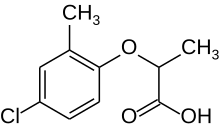
| |
| Names | |
|---|---|
| IUPAC name
(RS)-2-(4-Chloro-2-methylphenoxy)propanoic acid
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.002.060 |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C10H11ClO3 | |
| Molar mass | 214.65 g·mol−1 |
| Appearance | Solid |
| Melting point | 94 to 95 °C (201 to 203 °F; 367 to 368 K) [2] |
| Boiling point | decomposes [2] |
| 900 mg/L (20 °C)[2] | |
| Hazards | |
| Main hazards | Xn, N [2] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Mecoprop (also known as methylchlorophenoxypropionic acid and MCPP) is a common general use herbicide found in many household weed killers and "weed-and-feed" type lawn fertilizers.[3] It is primarily used to control broadleaf weeds.[4] It is often used in combination with other chemically related herbicides such as 2,4-D, dicamba, and MCPA.
The United States Environmental Protection Agency has classified mecoprop as toxicity class III - slightly toxic.[4]
Mecoprop is a mixture of two stereoisomers, with the (R)-(+)-enantiomer ("Mecoprop-P", "Duplosan KV") possessing the herbicidal activity.[5]

Structures of the two enantiomeric forms (S left, R right) of mecoprop
See also[]
- Clofibric acid
- Phenoxy herbicides
References[]
- ^ Merck Index, 11th Edition, 5666.
- ^ a b c d Record of Mecoprop in the GESTIS Substance Database of the Institute for Occupational Safety and Health, accessed on 8 September 2008.
- ^ Record in the Household Products Database of NLM
- ^ a b Mecoprop at EXTOXNET
- ^ G. Smith; C. H. L. Kennard; A. H. White; P. G. Hodgson (April 1980). "(±)-2-(4-Chloro-2-methylphenoxy)propionic acid (mecoprop)". Acta Crystallogr. B. 36 (4): 992–994. doi:10.1107/S0567740880005134.
External links[]
- Mecoprop Pesticide Information Profile - Extension Toxicology Network
- Mecoprop in the Pesticide Properties DataBase (PPDB)
- Mecoprop-P in the Pesticide Properties DataBase (PPDB)
Categories:
- Auxinic herbicides
- Chloroarenes
- Carboxylic acids