Indaziflam
| |
| Names | |
|---|---|
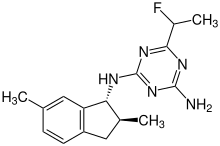
| IUPAC name
2-N-[(1R,2S)-2,6-dimethyl-2,3-dihydro-1H-inden-1-yl]-6-(1-fluoroethyl)-1,3,5-triazine-2,4-diamine[1]
| |
| Identifiers | |
3D model (JSmol)
|
|
| 20920435 | |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.216.692 |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| Properties[2] | |
| C16H20FN5 | |
| Molar mass | 301.369 g·mol−1 |
| Density | 1.23 g/mL |
| Melting point | 183 °C (361 °F; 456 K) |
| 2.8 mg/L (20 °C) | |
| log P | 2.8 |
| Hazards | |
| GHS pictograms |  
|
| GHS Signal word | Warning |
GHS hazard statements
|
H373, H400, H410 |
| P260, P273, P314, P391, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Indaziflam is a preemergent herbicide especially for grass control in tree and bush crops.
History[]
In 1991, the Japanese company Idemitsu Kosan filed a patent to 2-amino 6-fluoroalkyl triazine derivatives as herbicides.[3] One of these compounds was subsequently given the ISO common name triaziflam but had limited success as a commercial herbicide.[4][5] Bayer scientists subsequently investigated this area of chemistry and identified indaziflam as having superior properties, which they patented and developed under the code number BCS-AA10717.[6][7] The compound was first registered for use in the USA in 2010.[8][9]
Mechanism of action[]
Indaziflam is an inhibitor of cellulose biosynthesis. This mechanism of action was theorized to be responsible for indaziflam's effect in 2009[7] and proven in 2014.[10] The (CBIs) are identified as Class 29 by the Weed Science Society of America/Herbicide Resistance Action Committee.[11][12]
Resistance[]
As of March 2021 there are no resistant populations known[13] and none for the broader CBI class (discounting quinclorac).[11][14][15][16][17]
Brand names[]
Indaziflam composes all or part of the a.i. of several herbicides from Bayer Environmental Science, including the Esplanade[18] line (sometimes mixed with diquat dibromide and glyphosate isopropylamine),[19] Marengo,[20][21] Specticle,[22][21] and Bayer CropScience (the inventor of the ingredient), like Alion.[23]
Uses[]
Indaziflam is approved in the United States for hops, Rubus spp., Coffea spp., bushberries, tropical crops, drupes/stone fruit, and tree nuts.[24] It is used as a preemergent.[25][24]
References[]
- ^ Weed Science Society of America. "Common and chemical names approved by WSSA" (PDF).
- ^ Pesticide Properties Database. "Indaziflam". University of Hertfordshire.
- ^ US patent 5169425, Takematsu T.; Hirata T. & Kobayashi I. et al., "Herbicidal compositions comprising 2-Amino-4-Arylalkylamino-6-Haloalkyl-1,3,5-Triazines and Chlorophenoxy Acids and, optionally, substituted ureas", issued 1992-12-08, assigned to Idemitsu Kosan Company Limited
- ^ Grossmann, Klaus; Tresch, Stefan; Plath, Peter (2001). "Triaziflam and Diaminotriazine Derivatives Affect Enantioselectively Multiple Herbicide Target Sites". Zeitschrift für Naturforschung C. 56 (7–8): 559–569. doi:10.1515/znc-2001-7-814. PMID 11531090. S2CID 13128483.
- ^ "Triaziflam (ISO)". chem.nlm.nih.gov. Retrieved 2021-03-21.
- ^ WO patent 2004069814, Ahrens H.; Dietrich H. & Minn K. et al., "Amino 1,3,5-Triazines N-substituted with chiral bicyclic radicals", issued 2004-08-19, assigned to Bayer Cropscience GMBH
- ^ Jump up to: a b Meyer DF, Hanrahan R, Michel J, Monke B, Mudge L, Norton L, Olsen C, Parker A, Smith J, Spak D (2009). Indaziflam/BCS-AA10717-A new herbicide for pre-emergent control of grasses and broadleaf weeds for turf and ornamentals. WSSA Meeting Abstracts.CS1 maint: uses authors parameter (link)
- ^ Mark D. Parrish, R. Darren Unland, and William J. Bertges, Bayer CropScience, Research Triangle Park, NC (7–10 December 2009). "Introduction of Indaziflam for Weed Control in Fruit, Nut, and Grape Crops". North Central Weed Science Society Proceedings. Kansas City, Mo: . 64: 164.CS1 maint: uses authors parameter (link) CS1 maint: date format (link)
- ^ "Bayer CropSciences new herbicide indaziflam received first registration in U.S." Grainews. 2010-09-06. Retrieved 2021-03-14.
- ^ Brabham, C.; Lei, L.; Gu, Y.; Stork, J.; Barrett, M.; DeBolt, S. (2014-07-30). "Indaziflam Herbicidal Action: A Potent Cellulose Biosynthesis Inhibitor". Plant Physiology. American Society of Plant Biologists (OUP). 166 (3): 1177–1185. doi:10.1104/pp.114.241950. ISSN 0032-0889. PMC 4226351. PMID 25077797. S2CID 12250466.
- ^ Jump up to: a b Weed Science Society of America (2020-12-03). "WSSA-Herbicide Site of Action (SOA) Classification List".
- ^ Weed Science Society of America. "Summary of Herbicide Mechanism of Action According to the Weed Science Society of America (WSSA)" (PDF).
- ^ "Herbicide Resistant Weeds by Individual Herbicide". International Survey of Herbicide Resistant Weeds. Herbicide Resistance Action Committee. Retrieved 2021-03-14.
- ^ "List of Herbicide Resistant Weeds by Herbicide Mode of Action (L/26)". International Survey of Herbicide Resistant Weeds. Herbicide Resistance Action Committee. Retrieved 2021-03-14.
- ^ Grossmann, Klaus; Kwiatkowski, Jacek (2000). "The Mechanism of Quinclorac Selectivity in Grasses". Pesticide Biochemistry and Physiology. Elsevier. 66 (2): 83–91. doi:10.1006/pest.1999.2461. ISSN 0048-3575. S2CID 84092985.
- ^ Tresch, Stefan; Grossmann, Klaus (2003). "Quinclorac does not inhibit cellulose (cell wall) biosynthesis in sensitive barnyard grass and maize roots". Pesticide Biochemistry and Physiology. Elsevier. 75 (3): 73–78. doi:10.1016/s0048-3575(03)00013-0. ISSN 0048-3575. S2CID 84212641.
- ^ Tresch, Stefan; Grossmann, Klaus (2003). "Erratum to "Quinclorac does not inhibit cellulose (cell wall) biosynthesis in sensitive barnyard grass and maize roots"". Pesticide Biochemistry and Physiology. Elsevier. 76 (2): 70–71. doi:10.1016/s0048-3575(03)00064-6. ISSN 0048-3575. S2CID 84794877.
- ^ "Esplanade 200 SC IVM Product". Bayer Environmental Science US. Retrieved 2021-03-14.
- ^ "Esplanade EZ IVM Product". Bayer Environmental Science US. Retrieved 2021-03-14.
- ^ Spesard, Bruce (2018-06-08). "Broadleaf and Grassy Weed Control". Bayer Environmental Science. Retrieved 2021-03-14.
- ^ Jump up to: a b "Marengo (indaziflam) or Specticle". Extension Publications. NC State Ag Extension. 2014-05-30. Retrieved 2021-03-14.
- ^ "Specticle Flo". Bayer Environmental Science. Retrieved 2021-03-14.
- ^ "US84467332F (170705Fv2) ALION SC 32 FOZ ETL 0119.indd". CropScience. Bayer. 2019-11-01.
- ^ Jump up to: a b "PRIA Label Amendment – IR-4 tolerance petition and related amendments: (R190) to establish new uses on Hops, Caneberry subgroup 13-07A, Coffee, Bushberry subgroup 13-07B, Tropicals 23A and another amendment, and (R175) for crop group conversions in Stone Fruits 12-12 and Tree Nuts 14-12 Product Name: Indaziflam 200 SC Herbicide EPA Registration Number: 264-1106 Petition Number: 6E8452 Application Date: February 18, 2016 Decision Number(s): 514431, 514432, 514435, 514436" (PDF). US EPA. 2017-07-05.
- ^ "Indaziflam". PubChem. NCBI, NLM, US NIH. Retrieved 2021-03-14. CID=CID 44146693 from PubChem
- Agriculture stubs
- Herbicides
- Triazines
- Fluoroalkanes
- Amines
