Methanesulfonic acid
| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
Methanesulfonic acid | |
| Other names
Methylsulfonic acid, MSA
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.000.817 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| Properties | |
| CH4O3S | |
| Molar mass | 96.10 g·mol−1 |
| Appearance | Clear, colourless liquid |
| Density | 1.48 g/cm3 |
| Melting point | 17 to 19 °C (63 to 66 °F; 290 to 292 K) |
| Boiling point | 167 °C (333 °F; 440 K) at 10 mmHg, 122 °C/1 mmHg |
| miscible | |
| Solubility | Miscible with methanol, diethyl ether. Immiscible with hexane |
| log P | -2.424[1] |
| Acidity (pKa) | −1.9[2] |
| Hazards | |
| Safety data sheet | Oxford MSDS |
EU classification (DSD) (outdated)
|
Harmful (Xn), Corrosive (C) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
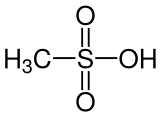
Methanesulfonic acid (MsOH) or methanesulphonic acid (in British English) is a colorless liquid with the chemical formula CH3SO3H. It is the simplest of the alkylsulfonic acids. Salts and esters of methanesulfonic acid are known as mesylates (or methanesulfonates, as in ethyl methanesulfonate). It is hygroscopic in its concentrated form. Methanesulfonic acid may be considered an intermediate compound between sulfuric acid (H2SO4), and methylsulfonylmethane ((CH3)2SO2), effectively replacing an –OH group with a –CH3 group at each step. This pattern can extend no further in either direction without breaking down the –SO2– group. Methanesulfonic acid can dissolve a wide range of metal salts, many of them in significantly higher concentrations than in hydrochloric or sulfuric acid.[3]
Applications[]
Methanesulfonic acid is used as an acid catalyst in organic reactions because it is a non-volatile, strong acid that is soluble in organic solvents. It is convenient for industrial applications because it is liquid at ambient temperature, while the closely related p-toluenesulfonic acid (PTSA) is solid. However, in a laboratory setting, solid PTSA is more convenient.
Methanesulfonic acid can be used in the generation of borane (BH3) by reacting methanesulfonic acid with NaBH4 in an aprotic solvent such as THF or DMS, the complex of BH3 and the solvent is formed.[4]
Electroplating[]
Solutions of methanesulfonic acid are used for the electroplating of tin and tin-lead solders. It is displacing the use of fluoroboric acid, which releases corrosive and volatile hydrogen fluoride.[5]
Methanesulfonic acid is also a primary ingredient in rust and scale removers.[6] It is used to clean off surface rust from ceramic, tiles and porcelain which are usually susceptible to acid attack.
See also[]
- Trifluoromethanesulfonic acid - the more acidic trifluoro analogue
References[]
- ^ Towler, Christopher S.; Li, Tonglei; Wikström, Håkan; Remick, David M.; Sanchez-Felix, Manuel V.; Taylor, Lynne S. (December 2008). "An Investigation into the Influence of Counterion on the Properties of Some Amorphous Organic Salts". Molecular Pharmaceutics. 5 (6): 946–955. doi:10.1021/mp8000342. PMID 19434850.
- ^ Guthrie, J. Peter (September 1978). "Hydrolysis of esters of oxy acids: pKa values for strong acids; Brønsted relationship for attack of water at methyl; free energies of hydrolysis of esters of oxy acids; and a linear relationship between free energy of hydrolysis and pKa holding over a range of 20 pK units". Canadian Journal of Chemistry. 56 (17): 2342–2354. doi:10.1139/v78-385.
- ^ Gernon, M. D.; Wu, M.; Buszta, T.; Janney, P. (1999). "Environmental benefits of methanesulfonic acid: comparative properties and advantages". Green Chemistry. 1 (3): 127–140. doi:10.1039/a900157c.
- ^ Lobben, Paul C.; Leung, Simon Shun-Wang; Tummala, Srinivas (2004). "Integrated Approach to the Development and Understanding of the Borane Reduction of a Carboxylic Acid". Org. Process Res. Dev. 8: 1072. doi:10.1021/op049910h.
- ^ Balaji, R.; Pushpavanam, Malathy (2003). "Methanesulphonic acid in electroplating related metal finishing industries". Transactions of the Imf. 81 (5): 154–158. doi:10.1080/00202967.2003.11871526. S2CID 91584456.
- ^ "Archived copy" (PDF). Archived from the original (PDF) on 2016-03-04. Retrieved 2015-12-01.CS1 maint: archived copy as title (link)
- Sulfonic acids
- Reagents for organic chemistry
- Acid catalysts