Methanetetracarboxylate
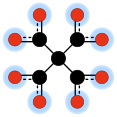 Dots that are highlighted in blue carry a partial negative charge.
| |
| Names | |
|---|---|
| Preferred IUPAC name
Methanetetracarboxylate[PubChem 1] | |
| Identifiers | |
| |
3D model (JSmol)
|
|
PubChem CID
|
|
| |
| |
| Properties | |
| C5O84− | |
| Molar mass | 188.049 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
In chemistry, methanetetracarboxylate is a tetravalent anion with formula C
5O4−
8 or C(COO−)4. It has four carboxylate groups attached to a central carbon atom; so it has the same carbon backbone as neopentane. It is an oxocarbon anion, that is, consists only of carbon and oxygen.
The term is also used for any salt with that anion; or for any ester with the C(COO)4 moiety.[1]
The salts and esters are relatively uncommon, and their uses appear to be limited to chemical research. The Na4C(COO)4 can be obtained by oxidation of pentaerythritol C(CH2OH)4 with oxygen in sodium hydroxide solution at pH 10 and about 60 °C, in the presence of palladium as a catalyst.[2]
The anion can be seen as the result of removing four protons from methanetetracarboxylic acid, a hypothetical organic compound with formula C5H4O8 or C(COOH)4. This acid has not been synthesised (as of 2009), and is believed to be unstable, but its , C(COOCH2CH3)4, is a specialty chemical and has been used in organic synthesis.[3]
References[]
- ^ Backer, H. J.; Lolkema, J. (1939). "Les Éthers de L'acide Méthanetétracarboxylique" [The Esters of Methanetetracarboxylic Acid]. Recueil des Travaux Chimiques des Pays-Bas (in French). 58: 23–33. doi:10.1002/recl.19390580106.
- ^ WO patent 2012026212A1, 稲山俊宏 (Toshihiro INAYAMA); 聡日吉 (Satoshi HIYOSHI) & 信仁雨宮 (Nobuhito AMEMIYA) et al., "Tetraester of pentaerythritol", published 2012-03-01, assigned to Kyowa Hakko Kirin
- ^ de Meijere, Armin; Kostikov, Rafael R.; Savchenko, Andrei I.; Kozhushkov, Sergei I. (2004). "Diethyl Cyclopropylidenemalonate: Facile Preparation, Generation in situ, and Various Transformations". European Journal of Organic Chemistry. 2004 (19): 3992–4002. doi:10.1002/ejoc.200400374.
- ^ "NKVMCSDLYHGDMD-UHFFFAOYSA-J". pubchem.ncbi.nlm.nih.gov. Retrieved 14 March 2019.
IUPAC Name methanetetracarboxylate
- ^ "NKVMCSDLYHGDMD-UHFFFAOYSA-J". pubchem.ncbi.nlm.nih.gov. Retrieved 14 March 2019.
PubChem CID: 57459306
- ^ "SID 137126464 - PubChem". pubchem.ncbi.nlm.nih.gov. Retrieved 14 March 2019.
PubChem CID: 57459306
- ^ "NKVMCSDLYHGDMD-UHFFFAOYSA-J" (table). pubchem.ncbi.nlm.nih.gov. Retrieved 14 March 2019.
Source External ID PubChem SID chemicalize.org by ChemAxon 280211 137126464
- ^ "SID 137126464 - PubChem". pubchem.ncbi.nlm.nih.gov. Retrieved 14 March 2019.
PubChem SID: 137126464
- ^ "NKVMCSDLYHGDMD-UHFFFAOYSA-J". pubchem.ncbi.nlm.nih.gov. Retrieved 14 March 2019.
Canonical SMILES C(=O)(C(C(=O)[O-])(C(=O)[O-])C(=O)[O-])[O-]
- ^ "NKVMCSDLYHGDMD-UHFFFAOYSA-J". pubchem.ncbi.nlm.nih.gov. Retrieved 14 March 2019.
InChI InChI=1S/C5H4O8/c6-1(7)5(2(8)9,3(10)11)4(12)13/h(H,6,7)(H,8,9)(H,10,11)(H,12,13)/p-4
- ^ "NKVMCSDLYHGDMD-UHFFFAOYSA-J". pubchem.ncbi.nlm.nih.gov. Retrieved 14 March 2019.
InChI Key NKVMCSDLYHGDMD-UHFFFAOYSA-J
- Carboxylate anions
- Oxocarbons