PLGA
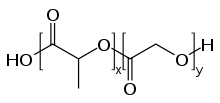
PLGA, PLG, or poly(lactic-co-glycolic acid) is a copolymer which is used in a host of Food and Drug Administration (FDA) approved therapeutic devices, owing to its biodegradability and biocompatibility. PLGA is synthesized by means of ring-opening co-polymerization of two different monomers, the cyclic dimers (1,4-dioxane-2,5-diones) of glycolic acid and lactic acid. Polymers can be synthesized as either random or block copolymers thereby imparting additional polymer properties. Common catalysts used in the preparation of this polymer include tin(II) 2-ethylhexanoate, tin(II) alkoxides, or aluminum isopropoxide. During polymerization, successive monomeric units (of glycolic or lactic acid) are linked together in PLGA by ester linkages, thus yielding a linear, aliphatic polyester as a product.[1]
Copolymer[]
Depending on the ratio of lactide to glycolide used for the polymerization, different forms of PLGA can be obtained: these are usually identified in regard to the molar ratio of the monomers used (e.g. PLGA 75:25 identifies a copolymer whose composition is 75% lactic acid and 25% glycolic acid). The crystallinity of PLGAs will vary from fully amorphous to fully crystalline depending on block structure and molar ratio. PLGAs typically show a glass transition temperature in the range of 40-60 °C. PLGA can be dissolved by a wide range of solvents, depending on composition. Higher lactide polymers can be dissolved using chlorinated solvents whereas higher glycolide materials will require the use of fluorinated solvents such as HFIP.
PLGA degrades by hydrolysis of its ester linkages in the presence of water. It has been shown that the time required for degradation of PLGA is related to the monomers' ratio used in production: the higher the content of glycolide units, the lower the time required for degradation as compared to predominantly lactide materials. An exception to this rule is the copolymer with 50:50 monomers' ratio which exhibits the faster degradation (about two months). In addition, polymers that are end-capped with esters (as opposed to the free carboxylic acid) demonstrate longer degradation half-lives.[2] This flexibility in degradation has made it convenient for fabrication of many medical devices, such as, grafts, sutures, implants, prosthetic devices, surgical sealant films, micro and nanoparticles.[3]
PLGA undergoes hydrolysis in the body to produce the original monomers: lactic acid and glycolic acid. These two monomers under normal physiological conditions, are by-products of various metabolic pathways in the body. Lactic acid is metabolized in the tricarboxylic acid cycle and eliminated via carbon dioxide and water. Glycolic acid is metabolized in the same way, and also excreted through the kidney.[4] Since the body can metabolize the two monomers, there is minimal systemic toxicity associated with using PLGA for biomaterial applications. However, it has been reported that the acidic degradation of PLGA reduces the local pH low enough to create an autocatalytic environment.[5] It has been shown that the pH inside a microsphere can become as acidic as pH 1.5.[6]
Examples[]
Specific examples of PLGA's use include:
- A commercially available drug delivery device using PLGA is Lupron Depot for the treatment of advanced prostate cancer.
- prophylactic delivery of the antibiotic vancomycin into the central nervous system when applied to the surface of the brain after brain surgery[7][8]
See also[]
- Polycaprolactone
- Polyglycolide
- Polymer-drug conjugates
- Polylactic acid
- Poly-3-hydroxybutyrate
References[]
- ^ Astete, C. E. & Sabliov, C. M. (2006). "Synthesis and characterization of PLGA nanoparticles". Journal of Biomaterials Science, Polymer Edition. 17 (3): 247–289. doi:10.1163/156856206775997322. PMID 16689015.
- ^ Samadi, N.; Abbadessa, A.; Di Stefano, A.; van Nostrum, C. F.; Vermonden, T.; Rahimian, S.; Teunissen, E. A.; van Steenbergen, M. J.; Amidi, M. & Hennink, W. E. (2013). "The effect of lauryl capping group on protein release and degradation of poly(D,L-lactic-co-glycolic acid) particles". Journal of Controlled Release. 172 (2): 436–443. doi:10.1016/j.jconrel.2013.05.034. PMID 23751568.
- ^ Pavot, V; Berthet, M; Rességuier, J; Legaz, S; Handké, N; Gilbert, SC; Paul, S; Verrier, B (December 2014). "Poly(lactic acid) and poly(lactic-co-glycolic acid) particles as versatile carrier platforms for vaccine delivery". Nanomedicine (Lond.). 9 (17): 2703–18. doi:10.2217/nnm.14.156. PMID 25529572.
- ^ Crotts, G (2 July 1998). "Protein delivery from poly(lactic-co-glycolic acid) biodegradable microspheres: Release kinetics and stability issues". Journal of Microencapsulation. 15 (6): 699–713. doi:10.3109/02652049809008253. PMID 9818948.
- ^ Zolnik, Banu; Burgess, Diane (2007). "Effect of acidic pH on PLGA microsphere degradation and release". JCR. 122 (3): 338–44. doi:10.1016/j.jconrel.2007.05.034. PMID 17644208.
- ^ Karen, Fu; Pack, Daniel; Alexander, Klibanov; Langer, Robert (2000). "Visual Evidence of Acidic Environment Within Degrading Poly(lactic-co-glycolic acid) (PLGA) Microspheres". Pharm Res. 17 (1): 100–106. doi:10.1023/A:1007582911958. PMID 10714616.
- ^ "Dissolvable Plastic Nanofibers could Treat Brain Infections". Scientific Computing. Advantage Business Media. August 28, 2013. Retrieved September 3, 2013.
- ^ "PLGA 50 50". Scientific Computing. Advantage Business Media. August 28, 2013. Retrieved September 3, 2013.
External links[]
 Media related to PLGA at Wikimedia Commons
Media related to PLGA at Wikimedia Commons
- Copolymers
- Synthetic fibers
- Biodegradable plastics
- Polyesters