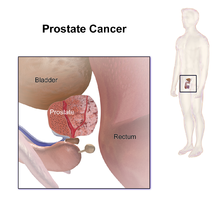
Prostate cancer
| Prostate cancer | |
|---|---|
| Other names | Carcinoma of the prostate |
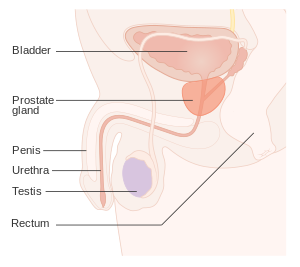 | |
| Position of the prostate | |
| Specialty | Oncology, urology |
| Symptoms | None, difficulty urinating, blood in the urine, pain in the pelvis, back, or when urinating[1][2] |
| Usual onset | Age > 50[3] |
| Risk factors | Older age, family history, race[3] |
| Diagnostic method | Tissue biopsy, medical imaging[2] |
| Differential diagnosis | Benign prostatic hyperplasia[1] |
| Treatment | Active surveillance, surgery, radiation therapy, hormone therapy, chemotherapy[2] |
| Prognosis | 5-year survival rate 99% (US)[4] |
| Frequency | 1.2 million new cases (2018)[5] |
| Deaths | 359,000 (2018)[5] |
Prostate cancer is cancer of the prostate. The prostate is a gland in the male reproductive system that surrounds the urethra just below the bladder.[6] Most prostate cancers are slow growing.[1][3] Cancerous cells may spread to other areas of the body, particularly the bones and lymph nodes.[7] It may initially cause no symptoms.[1] In later stages, symptoms include pain or difficulty urinating, blood in the urine, or pain in the pelvis or back.[2] Benign prostatic hyperplasia may produce similar symptoms.[1] Other late symptoms include fatigue, due to low levels of red blood cells.[1]
Factors that increase the risk of prostate cancer include older age, family history and race.[3] About 99% of cases occur after age 50.[3] A first-degree relative with the disease increases the risk two- to three-fold.[3] Other factors include a diet high in processed meat and red meat,[3] while the risk from a high intake of milk products is inconclusive.[8] An association with gonorrhea has been found, although no reason for this relationship has been identified.[9] An increased risk is associated with the BRCA mutations.[10] Diagnosis is by biopsy.[2] Medical imaging may be done to assess whether metastasis is present.[2]
Prostate cancer screening, including prostate-specific antigen (PSA) testing, increases cancer detection but whether it improves outcomes is controversial.[3][11][12][13] Informed decision making is recommended for those 55 to 69 years old.[14][15] Testing, if carried out, is more appropriate for those with a longer life expectancy.[16] Although 5α-reductase inhibitors appear to decrease low-grade cancer risk, they do not affect high-grade cancer risk, and are not recommended for prevention.[3] Vitamin or mineral supplementation does not appear to affect risk.[3][17]
Many cases are managed with active surveillance or watchful waiting.[2] Other treatments may include a combination of surgery, radiation therapy, hormone therapy, or chemotherapy.[2] Tumors limited to the prostate may be curable.[1] Pain medications, bisphosphonates, and targeted therapy,[18] among others, may be useful.[2] Outcomes depend on age, health status and how aggressive and extensive the cancer is.[2] Most men with prostate cancer do not die from it.[2] The United States five-year survival rate is 98%.[4]
Globally, it is the second-most common cancer. It is the fifth-leading cause of cancer-related death in men.[19] In 2018, it was diagnosed in 1.2 million and caused 359,000 deaths.[5] It was the most common cancer in males in 84 countries,[3] occurring more commonly in the developed world.[20] Rates have been increasing in the developing world.[20] Detection increased significantly in the 1980s and 1990s in many areas due to increased PSA testing.[3] One study reported prostate cancer in 30% to 70% of Russian and Japanese men over age 60 who had died of unrelated causes.[1]
Signs and symptoms[]
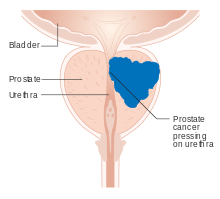
Early prostate cancer usually has no clear symptoms. When they do appear, they are often similar to those of benign prostatic hyperplasia. These include frequent urination, nocturia (increased urination at night), difficulty starting and maintaining a steady stream of urine, hematuria (blood in the urine), dysuria (painful urination) as well as fatigue due to anemia, and bone pain.[21] One study found that about a third of diagnosed patients had one or more such symptoms.[22]
Prostate cancer is associated with urinary dysfunction as the prostate gland surrounds the prostatic urethra. Changes within the gland directly affect urinary function. Because the vas deferens deposits seminal fluid into the prostatic urethra, and secretions from the prostate are included in semen content, prostate cancer may also cause problems with sexual function and performance, such as difficulty achieving erection or painful ejaculation.[22]
Metastatic prostate cancer can cause additional symptoms. The most common symptom is bone pain, often in the vertebrae (bones of the spine), pelvis, or ribs. Spread of cancer into other bones such as the femur is usually to the part of the bone nearer to the prostate. Prostate cancer in the spine can compress the spinal cord, causing tingling, leg weakness, and urinary and fecal incontinence.[23]
Risk factors[]
The primary risk factors are obesity,[24] age, and family history. Obese men have been found to have a 34% greater death rate from prostate cancer than those with normal weight.[24] Prostate cancer is uncommon in men younger than 45, but becomes more common with advancing age. The average age at the time of diagnosis is 70.[25] Autopsy studies of Chinese, German, Israeli, Jamaican, Swedish, and Ugandan men who died of other causes found prostate cancer in 30% of men in their 50s, and in 80% of men in their 70s.[26][27][28]
Men with high blood pressure are more likely to develop prostate cancer.[29] A small increase in risk is associated with lack of exercise.[30] Elevated blood testosterone levels[31] may increase risk.
Genetics[]
Genetics may affect risk, as suggested by associations with race, family, and specific gene variants.[32] Men who have a first-degree relative (father or brother) with prostate cancer have twice the risk of developing prostate cancer, and those with two first-degree relatives affected have a five-fold greater risk compared with men with no family history.[33][34] This risk appears to be greater for men with an affected brother than for those with an affected father. In the United States, prostate cancer more commonly affects black men than white or Hispanic men, and is also more deadly in black men.[35][36] In contrast, the incidence and mortality rates for Hispanic men are one-third lower than for non-Hispanic whites. Twin studies in Scandinavia suggest that 40% of prostate cancer risk can be explained by inherited factors.[37]
Many genes are involved in prostate cancer. Mutations in BRCA1 and BRCA2 (important risk factors for ovarian cancer and breast cancer in women) have been implicated.[38] Other linked genes include hereditary prostate cancer gene 1 (HPC1), the androgen receptor, and the vitamin D receptor.[35] TMPRSS2-ETS gene family fusion, specifically TMPRSS2-ERG or TMPRSS2-ETV1/4 promotes cancer cell growth.[39] These fusions can arise via complex rearrangement chains called chromoplexy.[40]
Two large genome-wide association studies linked single-nucleotide polymorphisms (SNPs) to prostate cancer in 2008.[41][42] These studies identified several relevant SNPs. For example, individuals with TT allele pair at SNP rs10993994 were reported to be at 1.6 times higher risk than those with the CC allele pair. This SNP explains part of the increased risk faced by African-Americans. The C allele is much more prevalent in the latter; this SNP is located in the promoter region of the MSMB gene, thus affects the amount of MSMB protein synthesized and secreted by epithelial cells of the prostate.[43]
While fewer studies have been conducted assessing the risk of being diagnosed with aggressive prostate cancer, a genome-wide association study (GWAS) of 12,518 prostate cancer cases identified two loci associated with high Gleason sum score, SNP rs78943174 nearest to the gene NAALADL2 and SNP rs35148638 nearest to RASA1.[44]
Dietary[]
Consuming fruits and vegetables has been found to be of little preventive benefit.[45] Some studies report that higher meat consumption was associated with higher risk.[46]
The consumption of milk may be related to prostate cancer.[47][48] A 2020 systematic review found the results on milk consumption and prostate cancer inconclusive but stated that individuals with higher risk may reduce or eliminate milk.[49] A 2019 overview stated that the evidence that linked milk to higher rates of prostate cancer was inconsistent and inconclusive.[50]
Lower blood levels of vitamin D may increase risks.[51] One study found no effect of folic acid supplements on risk.[52]
Medication exposure[]
Some links have been established between prostate cancer and medications, medical procedures, and medical conditions.[53] Statins may also decrease risk.[54]
Infection[]
Prostatitis (infection or inflammation) may increase risk. In particular, infection with the sexually transmitted infections Chlamydia, gonorrhea, or syphilis seems to increase risk.[9][55]
Papilloma virus has been proposed to have a potential role, but as of 2015, the evidence was inconclusive;[56] as of 2018, the increased risk was debated.[57]
Environment[]
US war veterans who had been exposed to Agent Orange had a 48% increased risk of prostate cancer recurrence following surgery.[medical citation needed]
Sex[]
Although some evidence from prospective cohort studies indicates that frequent ejaculation may reduce prostate cancer risk,[58] no randomized controlled trials reported this benefit.[59] An association between vasectomy and prostate cancer was found, but causality has not been established.[60]
Pathophysiology[]

The prostate is part of the male reproductive system that helps make and store seminal fluid. In adult men, a typical prostate is about 3 cm long and weighs about 20 g.[61] It is located in the pelvis, under the urinary bladder and in front of the rectum. The prostate surrounds part of the urethra, the tube that carries urine from the bladder during urination and semen during ejaculation.[62] The prostate contains many small glands, which make about 20% of the fluid constituting semen.[63]
Superiorly, the prostate base is contiguous with the bladder outlet. Inferiorly, the prostate's apex heads in the direction of the urogenital diaphragm, which is pointed anterio-inferiorly. The prostate can be divided into four anatomic spaces: peripheral, central, transitional, and anterior fibromuscular stroma.[64] The peripheral space contains the posterior and lateral portions of the prostate, as well as the inferior portions of the prostate. The central space contains the superior portion of the prostate including the most proximal aspects of the urethra and bladder neck. The transitional space is located just anterior to the central space and includes urethra distal to the central gland urethra. The neurovascular bundles course along the posterolateral prostate surface and penetrate the prostatic capsule there as well.
Most of the glandular tissue is found in the peripheral and central zones (peripheral zone: 70-80% of glandular tissue; central zone: 20% of glandular tissue).[65] Some is found in the transitional space (5% of glandular tissue). Thus, most cancers that develop from glandular tissue are found in the peripheral and central spaces,[66] while about 5% is found in the transitional space. None is found in the anterior fibromuscular stroma since no glands are in that anatomic space.
The prostate glands require male hormones, known as androgens, to work properly. Androgens include testosterone, which is made in the testes; dehydroepiandrosterone, made in the adrenal glands; and dihydrotestosterone, which is converted from testosterone within the prostate itself. Androgens are also responsible for secondary sex characteristics such as facial hair and increased muscle mass.
Because of the prostate's location, prostate diseases often affect urination, ejaculation, and rarely defecation. In prostate cancer, the cells of these glands mutate into cancer cells.


Most prostate cancers are classified as adenocarcinomas, or glandular cancers, that begin when semen-secreting gland cells mutate into cancer cells. The region of the prostate gland where the adenocarcinoma is most common is the peripheral zone. Initially, small clumps of cancer cells remain within otherwise normal prostate glands, a condition known as carcinoma in situ or prostatic intraepithelial neoplasia (PIN). Although no proof establishes that PIN is a cancer precursor, it is closely associated with cancer. Over time, these cells multiply and spread to the surrounding prostate tissue (the stroma) forming a tumor.
Eventually, the tumor may grow large enough to invade nearby organs such as the seminal vesicles or the rectum, or tumor cells may develop the ability to travel in the bloodstream and lymphatic system.
Prostate cancer is considered a malignant tumor because it can invade other areas of the body. This invasion is called metastasis. Prostate cancer most commonly metastasizes to the bones and lymph nodes, and may invade the rectum, bladder, and lower ureters after local progression. The route of metastasis to bone is thought to be venous, as the prostatic venous plexus draining the prostate connects with the vertebral veins.[67]
The prostate is a zinc-accumulating, citrate-producing organ. Transport protein ZIP1 is responsible for the transport of zinc into prostate cells. One of zinc's important roles is to change the cell's metabolism to produce citrate, an important semen component. The process of zinc accumulation, alteration of metabolism, and citrate production is energy inefficient, and prostate cells require enormous amounts of energy (ATP) to accomplish this task. Prostate cancer cells are generally devoid of zinc. Prostate cancer cells save energy by not making citrate, and use the conserved energy to grow, reproduce and spread.
The absence of zinc is thought to occur via silencing the gene that produces ZIP1. It is called a tumor suppressor gene product for the gene SLC39A1. The cause of the epigenetic silencing is unknown. Strategies that transport zinc into transformed prostate cells effectively eliminate these cells in animals. Zinc inhibits NF-κB pathways, is antiproliferative, and induces apoptosis in abnormal cells. Unfortunately, oral ingestion of zinc is ineffective since high concentrations of zinc into prostate cells is not possible without ZIP1.[68]
Loss of cancer suppressor genes, early in prostatic carcinogenesis, have been localized to chromosomes 8p, 10q, 13q, and 16q. P53 mutations in the primary prostate cancer are relatively low and are more frequently seen in metastatic settings, hence, p53 mutations are a late event in the pathology. Other tumor suppressor genes that are thought to play a role include PTEN and KAI1. "Up to 70 percent of men with prostate cancer have lost one copy of the PTEN gene at the time of diagnosis".[69] Relative frequency of loss of E-cadherin and CD44 has also been observed. Loss of the retinoblastoma (RB) protein induces androgen receptor deregulation in castration-resistant prostate cancer by deregulating 'E2F1 expression.[70]
RUNX2 is a transcription factor that prevents cancer cells from undergoing apoptosis, thereby contributing to cancer development.[71]
The PI3k/Akt signaling cascade works with the transforming growth factor beta/SMAD signaling cascade to ensure cancer cell survival and protect against apoptosis.[72] Pim-1 is upregulated in prostate cancer.[18] X-linked inhibitor of apoptosis (XIAP) is hypothesized to promote cancer cell survival and growth.[73] Macrophage inhibitory cytokine-1 (MIC-1) stimulates the focal adhesion kinase (FAK) signaling pathway which leads to cancer cell growth and survival.[74]
The androgen receptor helps cancer cells to survive.[75] Prostate-specific membrane antigen (PSMA) stimulates cancer development by increasing folate levels, helping the cancer cells to survive and grow; it increases available for use by hydrolyzing glutamated folates.[76]
Diagnosis[]

The American Cancer Society's position regarding early detection by PSA testing is:
Research has not yet proven that the potential benefits of testing outweigh the harms of testing and treatment. The American Cancer Society believes that men should not be tested without learning about what we know and don’t know about the risks and possible benefits of testing and treatment. Starting at age 50, (45 if African American or brother or father suffered from condition before age 65) talk to your doctor about the pros and cons of testing so you can decide if testing is the right choice for you."[77]
Several other tests can be used to gather information about the prostate and the urinary tract. Digital rectal examination may allow a doctor to detect prostate abnormalities. Cystoscopy shows the urinary tract from inside the bladder, using a thin, flexible camera tube inserted in the urethra. Transrectal ultrasonography creates a picture of the prostate using sound waves from a probe in the rectum, but the only test that can fully confirm the diagnosis of prostate cancer is a biopsy, the removal of small pieces of the prostate for microscopic examination.
Imaging[]
This section needs more medical references for verification or relies too heavily on primary sources. (March 2020) |
Ultrasound and magnetic resonance imaging (MRI) are the two main imaging methods used for prostate cancer detection.
MRI[]
Appearance of prostate on MRI[]
On MRI, the central and transitional zones both have lower T2 signal than the peripheral zone. Since the central and transitional zones cannot be distinguished from each other, they can be best described as the central gland on MRI. Thus, the peripheral gland has a higher signal on T2WI than the central gland. In the peripheral gland, prostate cancer appears as a low-intensity lesion. However, in the central gland, low-intensity lesions cannot be distinguished from the low-intensity central gland. Diffusion restriction is instrumental in identifying and characterizing central gland lesions. Combined diffusion-weighted (DW) imaging and dynamic contrast-enhanced MRI for distinguish malignant from benign prostate lesions can be used. The merged images, of DW and MRI with dynamic contrast enhancement, can visualise areas with low signal intensity and fast wash-out effect - characteristic of carcinomas.[78] Lymphadenopathy can be seen best on postcontrast, fat-suppressed T1WI. Other regions can be described on MRI. The anterior fibromuscular stroma and the prostate capsule along the posterior and lateral prostate have a low T2WI signal, in contrast with the bright signal of the peripheral zone. Extraprostatic extension can be seen with disruption of capsule integrity.
MRI for the detection of prostate cancer[]
As of 2011, MRI was used to identify targets for prostate biopsy using fusion MRI with ultrasound (US) or MRI-guidance alone. An MRI alone will correctly identify 91% of men with clinically significant prostate cancer but will misclassify 63% of men at risk for prostate cancer as having clinically significant prostate cancer.[79] An MRI-targeted biopsy will correctly identify 80% of men with prostate cancer. However, it will classify 6% of men at risk for prostate cancer as having clinically significant prostate cancer.[79]
Following an MRI, regions of interest within the scan which may be cancer are often graded on a likelihood scale between 1 and 5. One such scale is the prostate imaging-reporting and data system (PI-RADS) scale which defines standards of clinical service for multiparametric MRI (mpMRI), including image creation and reporting. PI-RADS version 2 scoring has shown a specificity and sensitivity of 73% and 95%, respectively, for detection of prostate cancer.[80]
When an MRI is used to decide whether to do a biopsy in men who have had a prior biopsy, it is 5% more likely to make a correct diagnosis than a standard biopsy and is 12% more likely to be correct for men who may or may not have had a prior biopsy.[79] In men who have had a negative biopsy, this combination is 44% more likely to lead to a correct diagnosis.[79]
Other uses for MRI[]
Prostate MRI is also used for surgical planning for robotic prostatectomy. It helps surgeons decide whether to resect or spare the neurovascular bundle, determine return to urinary continence, and help assess surgical difficulty.[81] MRI is used in other types of treatment planning, for both focal therapy[82] and radiotherapy.[83] MRI can also be used to target areas for research sampling in biobanking.[84][85]
Biological basis for prostate cancer visibility on MRI[]
The biological properties which determine whether or not a tumour is visible on MRI is poorly understood. One theory is that tumour cells undergo several genetic changes during transformation which alter the cellular rate of growth and formation of new blood vessels, leading to tumours with more aggressive histological patterns, hypoxic regions and increased cell density among other features.[86] Having larger, more dense tumours with changes in blood vessel distributions may feasibly alter signal on MRI through restriction of water and/or fluid movement.[86]
Some studies have linked the presence of rare histological patterns within the tumour such as cribriform pattern.[87] Although recent research suggests there is a number of histopathological features which may influence tumour detection by MRI.[88] At a genetic level, prostate cancer visibility on MRI seems to be linked with genetic features of aggressive disease including processes such as cell proliferation, tumour hypoxia and DNA damage.[89] The gene changes consistently observed in MRI-visible tumours include loss of tumour suppressor PTEN, increased expression of proliferation associated genes CENPF, AGR2 and growth factor GDF15 as well as a number of other genes.[89] Changes in these pathways and genes may facilitate increased tumour growth, changes in vasculature and density which ultimately change the signal on MRI.[86]
Ultrasound[]
Ultrasound imaging can be obtained transrectally and is used during prostate biopsies. Prostate cancer can be seen as a hypoechoic lesion in 60% of cases. The other 40% of cancerous lesions are either hyperechoic or isoechoic. On Color Doppler, the lesions appear hypervascular.
Biopsy[]

If cancer is suspected, a biopsy is offered expediently. During a biopsy, a urologist or radiologist obtains tissue samples from the prostate via either the rectum or the perineum.[failed verification] A biopsy gun inserts and removes special hollow-core needles (usually three to six on each side of the prostate) in less than a second. Prostate biopsies are routinely done on an outpatient basis and rarely require hospitalization. Systematic biopsies correctly identify 63% of men as having clinically significant prostate cancer but will miss the rest.[79][90] For men at risk for prostate cancer, biopsy will not misclassify any of the men as having clinically significant prostate cancer.[79][90]
Antibiotics should be used to prevent complications such as fever, urinary tract infections, and sepsis[91] even if the most appropriate course or dose is undefined.[92] About 55% of men report discomfort during prostate biopsy.[93]
Histopathologic diagnosis[]


A histopathologic diagnosis mainly includes assessment of whether a cancer exists, as well as any subdiagnosis, if possible. Histopathologic subdiagnosis has implications for the possibility and methodology of Gleason scoring.[95] The most common histopathological subdiagnosis is acinar adenocarcinoma, constituting 93% of diagnoses.[96] The most common form of acinar adenocarcinoma, in turn, is "adenocarcinoma, not otherwise specified", also termed conventional, or usual acinar adenocarcinoma.[97]
Biochemical diagnosis[]
Alkaline phosphatase is more elevated in metastatic than non-metastatic cells.[98] High levels of alkaline phosphatase is associated with a significant decrease in survival.[98]
Gleason score[]
The Gleason grading system is used to help evaluate the prognosis and helps guide therapy. A Gleason score is based upon the tumor's appearance.[99] Cancers with a higher Gleason score are more aggressive and have a worse prognosis. Pathological scores range from 2 through 10, with a higher number indicating greater risks and higher mortality.
Tumor markers[]
Tissue samples can be stained for the presence of PSA and other tumor markers to determine the origin of malignant cells that have metastasized.[100]
Small cell carcinoma is a rare (1%[101]) type that cannot be diagnosed using PSA.[101][102] As of 2009 researchers were investigating ways to screen for this type, because it is quick to metastasize.[102]
The oncoprotein BCL-2 is associated with the development of androgen-independent prostate cancer, due to its high levels of expression in androgen-independent tumours in advanced stages. The upregulation of BCL-2 after androgen ablation in prostate carcinoma cell lines and in a castrated-male rat model further established a connection between BCL-2 expression and prostate cancer progression.[103]
Staging[]

An important part of evaluating prostate cancer is determining the stage, or degree of spread. Knowing the stage helps define prognosis and is useful when selecting therapies. The most common system is the four-stage TNM system (abbreviated from tumor/nodes/metastases). Its components include the size of the tumor, the number of involved lymph nodes, and the presence of any other metastases.[104]
The most important distinction made by any staging system is whether the cancer is confined to the prostate. In the TNM system, clinical T1 and T2 cancers are found only in the prostate, while T3 and T4 cancers have metastasized. Several tests can be used to look for evidence of spread. Medical specialty professional organizations recommend against the use of PET scans, CT scans, or bone scans when a physician stages early prostate cancer with low risk for metastasis.[105] Those tests would be appropriate in cases such as when a CT scan evaluates spread within the pelvis, a bone scan looks for spread to the bones, and endorectal coil magnetic resonance imaging evaluates the prostatic capsule and the seminal vesicles. Bone scans should reveal osteoblastic appearance due to increased bone density in the areas of bone metastasis—the reverse of what is found in many other metastatic cancers. Approved radiopharmaceutical diagnostic agents used in PET: fluciclovine (2016), (2021).
After a biopsy, a pathologist examines the samples under a microscope. If cancer is present, the pathologist reports the grade of the tumor. The grade tells how much the tumor tissue differs from normal prostate tissue and suggests how fast the tumor is likely to grow. The pathologist assigns a Gleason number from 1 to 5 for the most common pattern observed under the microscope, then does the same for the second-most common pattern. The sum of these two numbers is the Gleason score. The Whitmore-Jewett stage is another method.
In men with high-risk localised prostate cancer, staging with PSMA PET/CT may be appropriate to detect nodal or distant metastatic spread. In 2020, a randomised phase 3 trial compared Gallium-68 PSMA PET/CT to standard imaging (CT and bone scan). It reported superior accuracy of Gallium-68 PSMA-11 PET/CT (92% vs 65%), higher significant change in management (28% vs 15%), less equivocal/uncertain imaging findings (7% vs 23%) and lower radiation exposure (10 msV vs 19 mSv). The study concluded that PSMA PET/CT is a suitable replacement for conventional imaging.[106]

Sclerosis of the bones of the thoracic spine due to prostate cancer metastases (CT image)
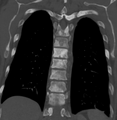
Sclerosis of the bones of the thoracic spine due to prostate cancer metastases (CT image)

Sclerosis of the bones of the pelvis due to prostate cancer metastases
Prevention[]
Diet and lifestyle[]
The data on the relationship between diet and prostate cancer are poor.[107] However, the rate of prostate cancer is linked to the consumption of the Western diet.[107] Little if any evidence associates trans fat, saturated fat, and carbohydrate intake and prostate cancer.[107][108] Evidence does not offer a role for omega-3 fatty acids in preventing prostate cancer.[107][109] Vitamin supplements appear to have no effect and some may increase the risk.[17][107] High supplemental calcium intake has been linked to advanced prostate cancer.[110]
Fish may lower prostate-cancer deaths, but does not appear to affect occurrence.[111] Some evidence supports lower rates of prostate cancer with a vegetarian diet,[112] lycopene, selenium[113][114] cruciferous vegetables, soy, beans and/or other legumes.[115]
Regular exercise may slightly lower risk, especially vigorous activity.[115]
Medications[]
In those who are regularly screened, 5-alpha-reductase inhibitors (finasteride and dutasteride) reduce the overall risk of prostate cancer. Data are insufficient to determine if they affect fatality risk and they may increase the chance of more serious cases.[116]
Screening[]
Prostate cancer screening searches for cancers in those without symptoms. Options include the digital rectal exam and the PSA blood test.[117] Such screening is controversial,[118] and for many, may lead to unnecessary disruption and possibly harmful consequences.[119] Harms of population-based screening, primarily due to overdiagnosis (the detection of latent cancers that would have otherwise not been discovered) may outweigh the benefits.[117] Others recommend shared decision-making, an approach where screening may occur after a physician consultation.[120]
The United States Preventive Services Task Force (USPSTF) suggests the decision whether to have PSA screening be based on consultation with a physician for men 55 to 69 years of age.[12] USPSTF recommends against PSA screening after age 70.[14] The Centers for Disease Control and Prevention endorsed USPSTF's conclusion.[121] The American Society of Clinical Oncology and the American College of Physicians discourage screening for those who are expected to live less than 10–15 years, while those with a greater life expectancy a decision should individually balance the potential risks and benefits.[122] In general, they concluded, "it is uncertain whether the benefits associated with PSA testing for prostate cancer screening are worth the harms associated with screening and subsequent unnecessary treatment."[123]
American Urological Association (AUA 2013) guidelines call for weighing the uncertain benefits of screening against the known harms associated with diagnostic tests and treatment. The AUA recommends that shared decision-making should control screening for those 55 to 69, and that screening should occur no more often than every two years.[124] In the United Kingdom as of 2015, no program existed to screen for prostate cancer.[13]
Management[]
The first decision is whether treatment is needed. Low-grade forms found in elderly men often grows so slowly that treatment is not required.[125] Treatment may also be inappropriate if a person has other serious health problems or is not expected to live long enough for symptoms to appear. Approaches in which treatment is postponed are termed "expectant management".[125] Expectant management is divided into two approaches: Watchful waiting, which has palliative intent (aims to treat symptoms only), and active surveillance, which has curative intent (aims to prevent the cancer from advancing).[125]
Which option is best depends on disease stage, the Gleason score, and the PSA level. Other important factors are age, general health and a person's views about potential treatments and their possible side effects. Because most treatments can have significant side effects, such as erectile dysfunction and urinary incontinence, treatment discussions often focus on balancing the goals of therapy with the risks of lifestyle alterations. A 2017 review found that more research focused on person-centered outcomes is needed to guide patients.[126] A combination of treatment options is often recommended.[127][128][129]
Although the widespread use of PSA screening in the US has resulted in diagnosis at earlier age and cancer stage, almost all cases are still diagnosed after age 65, while about 25% are diagnosed after age 75.[130] Though US National Comprehensive Cancer Network guidelines recommend using life expectancy to help make treatment decisions, in practice, many elderly patients are not offered curative treatment options such as radical prostatectomy or radiation therapy and are instead treated with hormonal therapy or watchful waiting.[131]
Guidelines for specific clinical situations require estimation of life expectancy.[132] As average life expectancy increases due to advances in the treatment of other diseases, more patients will live long enough for their prostate cancer to express symptoms. Therefore, interest grew in aggressive treatment modalities such as surgery or radiation even for localized disease.
Alternatively, an 18-item questionnaire was proposed to learn whether patients have adequate knowledge and understanding of their treatment options. In one 2015 study, most of those who were newly diagnosed correctly answered fewer than half of the questions.[132]
Surveillance[]
Many men diagnosed with low-risk prostate cancer are eligible for active surveillance. The tumor is carefully observed over time, with the intention of initiating treatment if signs of progression appear. Active surveillance is not synonymous with watchful waiting, a term which implies no treatment or specific program of monitoring, with the assumption that only palliative treatment would be used if advanced, symptomatic disease develops.[125]
Active surveillance involves monitoring the tumor for growth or symptoms, which trigger treatment. The monitoring process may involve PSA tests, digital rectal examination, and/or repeated biopsies every few months.[133] The goal of active surveillance is to postpone treatment, and avoid overtreatment and its side effects, given a slow-growing or self-limited tumor that in most people is unlikely to cause problems. This approach is not used for aggressive cancers, and may cause anxiety for people who wrongly believe that all cancers are deadly or that their condition is life-threatening. 50 to 75% of patients die from other causes without experiencing prostate symptoms.[134] In localized disease, based on long-term follow-up, radical prostatectomy results in significantly improved oncological outcomes when compared with watchful waiting.[135] However, there are also marked increases in rates of urinary incontinence and erectile dysfunction.[135] Since these results are based primarily on men diagnosed before widespread PSA screening, the results cannot be highly generalized.[135] When compared to active monitoring/surveillance, on follow-up at ten years, radical prostatectomy probably has similar outcomes for disease-specific survival and probably reduces risk of disease progression and spreading.[135] Still, urinary and sexual function are probably decreased in patients treated with radical prostatectomy.[135]
Active treatment[]
Both surgical and nonsurgical treatments are available, but treatment can be difficult, and combinations can be used.[136] Treatment by external beam radiation therapy, brachytherapy, cryosurgery, high-intensity focused ultrasound, and prostatectomy are, in general, offered to men whose cancer remains within the prostate. Hormonal therapy and chemotherapy are often reserved for metastatic disease. Exceptions include local or metastasis-directed therapy with radiation may be used for advanced tumors with limited metastasis.[137] Hormonal therapy is used for some early-stage tumors. Cryotherapy (the process of freezing the tumor), hormonal therapy, and chemotherapy may be offered if initial treatment fails and the cancer progresses. Sipuleucel-T, a cancer vaccine, was reported to offer a four-month increase in survival in metastatic prostate cancer,[138] but the marketing authorisation for it was withdrawn on 19 May 2015.
If radiation therapy fails, radical prostatectomy may be an option,[139] though it is a technically challenging surgery.[citation needed] However, radiation therapy after surgical failure may have many complications.[140] It is associated with a small increase in bladder and colon cancer.[141] Radiotherapy and surgery appear to result in similar outcomes with respect to bowel, erectile and urinary function after five years.[142]
Nonsurgical treatment[]
Non-surgical treatment may involve radiation therapy, chemotherapy, hormonal therapy, external beam radiation therapy, and particle therapy, high-intensity focused ultrasound, or some combination.[143][144]
Prostate cancer that persists when testosterone levels are lowered by hormonal therapy is called castrate-resistant prostate cancer (CRPC).[145][146] Many early-stage cancers need normal levels of testosterone to grow, but CRPC does not. Previously considered "hormone-refractory prostate cancer" or "androgen-independent prostate cancer", the term CRPC emerged because these cancers show reliance upon hormones, particularly testosterone, for androgen receptor activation.[147]
The cancer chemotherapeutic docetaxel has been used as treatment for CRPC with a median survival benefit of 2 to 3 months.[148][149] A second-line chemotherapy treatment is cabazitaxel.[150] A combination of bevacizumab, docetaxel, thalidomide and prednisone appears effective in the treatment of CRPC.[151]
Immunotherapy treatment with sipuleucel-T in CRPC appeared to increase survival by four months.[152] However, marketing authorisation for sipuleucel-T was withdrawn on 19 May 2015. The second line hormonal therapy abiraterone increases survival by about 4.6 months.[153] Enzalutamide is another second line hormonal agent with a five-month survival advantage. Both abiraterone and enzalutamide are currently in clinical trials in those with CRPC who have not previously received chemotherapy.[154][155]
Not all patients respond to androgen signaling-blocking drugs. Certain cells with characteristics resembling stem cells remain unaffected.[156][157] Therefore, the desire to improve CRPC outcomes resulted in increasing doses or combination therapy with synergistic androgen-signaling blocking agents.[158] But even these combination will not affect stem-like cells that do not exhibit androgen signaling.[159]
For patients with metastatic prostate cancer that has spread to their bones, doctors use a variety of bone-modifying agents to prevent skeletal complications and support the formation of new bone mass.[160] Zoledronic acid (a bisphosphonate) and denosumab (a RANK-ligand-inhibitor) appear to be effective agents, but are associated with more frequent and serious adverse events.[160]
Surgery[]
Radical prostatectomy is considered the mainstay of surgical treatment of prostate cancer, where the surgeon removes the prostate, seminal vesicles, and surrounding lymph nodes. It can be done by an open technique (a skin incision at the lower abdomen), or laparoscopically. Radical retropubic prostatectomy is the most commonly used open surgical technique.[citation needed] Robotic-assisted prostatectomy has become common.[161] Men with localized prostate cancer, having laparoscopic radical prostatectomy or robotic-assisted radical prostatectomy, might have shorter stays in the hospital and get fewer blood transfusions than men undergoing open radical prostatectomy.[162] How these treatments compare with regards to overall survival or recurrence-free survival is unknown.[162]
Transurethral resection of the prostate is the standard surgical treatment for benign enlargement of the prostate.[161] In prostate cancer, this procedure can be used to relieve symptoms of urinary retention caused by a large prostate tumor, but it is not used to treat the cancer itself. The procedure is done under spinal anesthesia, a resectoscope is inserted inside the penis and the extra prostatic tissue is cut to clear the way for the urine to pass.
Complications[]
The two main complications encountered after prostatectomy and prostate radiotherapy are erectile dysfunction and urinary incontinence, mainly stress-type. Most men regain continence within 6 to 12 months after the operation, so doctors usually wait at least one year before resorting to invasive treatments.[163]
Stress urinary incontinence usually happens after prostate surgery or radiation therapy due to factors that include damage to the urethral sphincter or surrounding tissue and nerves. The prostate surrounds the urethra, a muscular tube that closes the urinary bladder. Any of the mentioned reasons can lead to incompetent closure of the urethra and hence incontinence.[164] Initial therapy includes bladder training, lifestyle changes, kegel exercises, and the use of incontinence pads. More invasive surgical treatment can include the insertion of a urethral sling or an artificial urinary sphincter, which is a mechanical device that mimics the function of the urethral sphincter, and is activated manually by the patient through a switch implanted in the scrotum. The latter is considered the gold standard in patients with moderate or severe stress urinary incontinence.[165]
Erectile dysfunction happens in different degrees in nearly all men who undergo prostate cancer treatment, including radiotherapy or surgery; however, within one year, most of them will notice improvement. If nerves were damaged, this progress may not take place. Pharmacological treatment includes PDE-5 inhibitors such as viagra or cialis, or injectable intracavernous drugs injected directly into the penis (prostaglandin E1 and vasoactive drug mixtures). Other nonpharmacological therapy includes vacuum constriction devices and penile implants.[166]
Prognosis[]
Many prostate cancers are not destined to be lethal, and most men will ultimately not die as a result of the disease. Mortality varies widely across geography and other elements. In the United States, five-year survival rates range from 29% (distant metastases) to 100% (local or regional tumors).[167] In Japan, the fatality rate rose to 8.6/100,000 in 2000.[168] In India in the 1990s, half of those diagnosed with local cancer died within 19 years.[169] One study reported that African-Americans have 50–60 times more deaths than found in Shanghai, China.[170] In Nigeria, 2% of men develop prostate cancer, and 64% of them are dead after 2 years.[171] Most Nigerian men present with metastatic disease with a typical survival of 40 months.[172]
In patients who undergo treatment, the most important clinical prognostic indicators of disease outcome are the stage, pretherapy PSA level, and Gleason score. The higher the grade and the stage, the poorer the prognosis. Nomograms can be used to calculate the estimated risk of the individual patient. The predictions are based on the experience of large groups of patients.[173] A complicating factor is that the majority of patients have multiple independent tumor foci upon diagnosis, and these foci have independent genetic changes and molecular features.[174] Because of this extensive inter-focal heterogeneity, it is a risk that the prognostication is set based on the wrong tumor focus.
Androgen ablation therapy causes remission in 80–90% of patients undergoing therapy, resulting in a median progression-free survival of 12 to 33 months. After remission, an androgen-independent phenotype typically emerges, wherein the median overall survival is 23–37 months from the time of initiation of androgen ablation therapy.[175] How androgen-independence is established and how it re-establishes progression is unclear.[176]
Classification systems[]

Several tools are available to help predict outcomes, such as pathologic stage and recurrence after surgery or radiation therapy. Most combine stage, grade, and PSA level, and some include the number or percentage of biopsy cores positive, age, and/or other information.
- The D'Amico classification stratifies men by low, intermediate, or high risk based on stage, grade and PSA. It is used widely in clinical practice and research settings. The major downside to the three-level system is that it does not account for multiple adverse parameters (e.g., high Gleason score and high PSA) in stratifying patients.
- The Partin table][177] predict pathologic outcomes (margin status, extraprostatic extension, and seminal vesicle invasion) based on the same three variables and are published as lookup tables.
- The Kattan nomograms predict recurrence after surgery and/or radiation therapy, based on data available at the time of diagnosis or after surgery. The Kattan score represents the likelihood of remaining free of disease at a given time interval following treatment.
- The UCSF Cancer of the Prostate Risk Assessment (CAPRA) score predicts both pathologic status and recurrence after surgery. It offers accuracy comparable to the Kattan preoperative nomogram and can be calculated without tables or a calculator. Points are assigned based on PSA, grade, stage, age, and percentage of cores positive; the sum yields a 0–10 score, with every two points representing roughly a doubling of risk of recurrence. The CAPRA score was derived from community-based data in the CaPSURE database.[178] It has been validated among over 10,000 prostatectomy patients, including patients from CaPSURE;[179] the SEARCH registry, representing data from several Veterans Health Administration and military medical centers;[180] a multi-institutional cohort in Germany;[181] and the prostatectomy cohort at Johns Hopkins University.[182] More recently, it has been shown to predict metastasis and mortality following prostatectomy, radiation therapy, watchful waiting, or androgen deprivation therapy.[183]
Life expectancy[]
Life expectancy projections are averages for an entire male population, and many medical and lifestyle factors modify these numbers. For example, studies have shown that a 40-year-old man will lose 3.1 years of life if he is overweight (BMI 25–29) and 5.8 years of life if he is obese (BMI 30 or more), compared to men of normal weight. If he is both overweight and a smoker, he will lose 6.7 years, and if obese and a smoker, he will lose 13.7 years.[184]
No evidence shows that either surgery or beam radiation has an advantage over the other in this regard. The lower death rates reported with surgery appear to occur because surgery is more likely to be offered to younger men with less severe cancers. Insufficient information is available to determine whether seed radiation extends life more readily than the other treatments, but data so far do not suggest that it does.[185]
Men with low-grade disease (Gleason 2–4) were unlikely to die of prostate cancer within 15 years of diagnosis. Older men (age 70–75) with low-grade disease had a roughly 20% overall survival at 15 years due to deaths from competing causes. Men with high-grade disease (Gleason 8–10) experienced high mortality within 15 years of diagnosis, regardless of their age.[186]
Epidemiology[]

As of 2012, prostate cancer is the second-most frequently diagnosed cancer (at 15% of all male cancers)[188] and the sixth leading cause of cancer death in males worldwide.[189] In 2010, prostate cancer resulted in 256,000 deaths, up from 156,000 deaths in 1990.[190] Rates of prostate cancer vary widely. Rates vary widely between countries. It is least common in South and East Asia, and more common in Europe, North America, Australia, and New Zealand.[191] Prostate cancer is least common among Asian men and most common among black men, with white men in between.[192][193]
The average annual incidence rate of prostate cancer between 1988 and 1992 among Chinese men in the United States was 15 times higher than that of their counterparts living in Shanghai and Tianjin,[192][193][194] but these high rates may be affected by higher rates of detection.[195] Many suggest that prostate cancer may be under-reported, yet benign prostatic hyperplasia incidence in China and Japan is similar to rates in Western countries.[196][197]
More than 80% of men develop prostate cancer by age 80.[198]
United States[]
This section needs additional citations for verification. (August 2020) |

Prostate cancer is the third-leading cause of cancer death in men, exceeded by lung cancer and colorectal cancer. It accounts for 19% of all male cancers and 9% of male cancer-related deaths.
Cases ranged from an estimated 230,000 in 2005[199] to an estimated 164,690 In 2018.
Deaths held steady around 30,000 in 2005[199] and 29,430 in 2018.
Age-adjusted incidence rates increased steadily from 1975 through 1992, with particularly dramatic increases associated with the spread of PSA screening in the late 1980s, later followed by a fall in incidence. A decline in early-stage incidence rates from 2011 to 2012 (19%) in men aged 50 years and older persisted through 2013 (6%).
Declines in mortality rates in certain jurisdictions may reflect the interaction of PSA screening and improved treatment. The estimated lifetime risk is about 14.0%, and the lifetime mortality risk is 2.6%.
Between 2005 and 2011, the proportion of disease diagnosed at a locoregional stage was 93% for whites and 92% for African Americans; the proportion of disease diagnosed at a late stage was 4% for whites and 5% for African Americans.
Prostate cancer is more common in the African American population than the White American population.[3] An autopsy study of White and Asian men also found an increase in occult prostate cancer with age, reaching nearly 60% in men older than 80 years. More than 50% of cancers in Asian men and 25% of cancers in White men had a Gleason score of 7 or greater, suggesting that Gleason score may be an imprecise indicator of clinically insignificant cases.[200]
Canada[]
Prostate cancer is the third-leading type of cancer in Canadian men. In 2016, around 4,000 died and 21,600 men were diagnosed with prostate cancer.[118]
Europe[]
In Europe in 2012, it was the third-most diagnosed cancer after breast and colorectal cancers at 417,000 cases.[201]
In the United Kingdom, it is the second-most common cause of cancer death after lung cancer, where around 35,000 cases are diagnosed every year, of which around 10,000 are fatal.[202]
History[]
The prostate was first described by Venetian anatomist Niccolò Massa in 1536, and illustrated by Flemish anatomist Andreas Vesalius in 1538.[203] Prostate cancer was identified in 1853.[204][205] It was initially considered a rare disease, probably because of shorter life expectancies and poorer detection methods in the 19th century. The first treatments were surgeries to relieve urinary obstruction.[206]
Removal of the gland was first described in 1851,[207] and radical perineal prostatectomy was first performed in 1904 by Hugh H. Young at Johns Hopkins Hospital.[208][204]
Surgical removal of the testes (orchiectomy) to treat prostate cancer was first performed in the 1890s, with limited success. Transurethral resection of the prostate (TURP) replaced radical prostatectomy for symptomatic relief of obstruction in the middle of the 20th century because it could better preserve penile erectile function. Radical retropubic prostatectomy was developed in 1983 by Patrick Walsh.[209] This surgical approach allowed for removal of the prostate and lymph nodes with maintenance of penile function.
In 1941, Charles B. Huggins published studies in which he used estrogen to oppose testosterone production in men with metastatic prostate cancer. This discovery of "chemical castration" won Huggins the 1966 Nobel Prize in Physiology or Medicine.[210] The role of the gonadotropin-releasing hormone (GnRH) in reproduction was determined by Andrzej W. Schally and Roger Guillemin, who shared the 1977 Nobel Prize in Physiology or Medicine for this work. GnRH receptor agonists, such as leuprorelin and goserelin, were subsequently developed and used to treat prostate cancer.[211][212]
Radiation therapy for prostate cancer was first developed in the early 20th century and initially consisted of intraprostatic radium implants. External beam radiotherapy became more popular as stronger [X-ray] radiation sources became available in the middle of the 20th century. Brachytherapy with implanted seeds (for prostate cancer) was first described in 1983.[213]
Systemic chemotherapy for prostate cancer was first studied in the 1970s. The initial regimen of cyclophosphamide and 5-fluorouracil was quickly joined by regimens using other systemic chemotherapy drugs.[214]
Enzalutamide gained FDA approval in 2012 for the treatment of castration-resistant prostate cancer (CRPC).[154][155] Alpharadin won FDA approval in 2013, under the priority review program.[215]
In 2006, a previously unknown retrovirus, Xenotropic MuLV-related virus (XMRV), was associated with human prostate tumors,[216] but PLOS Pathogens retracted the article in 2012.[216]
Society and culture[]
Men with prostate cancer generally encounter significant disparities in awareness, funding, media coverage, and research—and therefore, inferior treatment and poorer outcomes—compared to other cancers of equal prevalence.[217] In 2001, The Guardian noted that Britain had 3,000 nurses specializing in breast cancer, compared to a single nurse for prostate cancer. Waiting time between referral and diagnosis was two weeks for breast cancer but three months for prostate cancer.[218]
A 2007 report by the U.S.-based National Prostate Cancer Coalition stated that prostate cancer drugs were outnumbered seven to one by breast cancer drugs. The Times also noted an "anti-male bias in cancer funding" with a four-to-one discrepancy in the United Kingdom by both the government and by cancer charities such as Cancer Research UK.[217][219] Critics cite such figures when claiming that women's health is favored over men's health.[220]
Disparities extend into detection, with governments failing to fund or mandate prostate cancer screening while fully supporting breast cancer programs. For example, a 2007 report found 49 U.S. states mandate insurance coverage for routine breast cancer screening, compared to 28 for prostate cancer.[221]
Prostate cancer experiences significantly less media coverage than other, equally prevalent cancers, outcovered 2.6:1 by breast cancer.[217]
Prostate Cancer Awareness Month takes place in September in a number of countries. A light blue ribbon is used to promote the cause.[222][223]
Research[]
Castration-resistant prostate cancer[]
Enzalutamide is a nonsteroidal antiandrogen (NSAA).[154][155]
Alpharadin uses bone targeted Radium-223 isotopes to kill cancer cells by alpha radiation.[224][unreliable medical source?]
PARP inhibitor olaparib is an approved breast/ovarian cancer drug that is undergoing clinical trials.[225] Also in trials for CRPC are : checkpoint inhibitor ipilimumab, CYP17 inhibitor galeterone (TOK-001), and immunotherapy PROSTVAC.[225]
All medications for CRPC block androgen receptor (AR) signaling via direct or indirect targeting of the AR ligand binding domain (LBD). AR belongs to the steroid nuclear receptor family. Development of the prostate is dependent on androgen signaling mediated through AR, and AR is also important for disease progression. Molecules that could successfully target alternative domains have emerged.[226] Such therapies could provide an advantage; particularly in treating prostate cancers that are resistant to current therapies.[226]
Pre-clinical[]
Arachidonate 5-lipoxygenase has been identified as playing a significant role in the survival of prostate cancer cells.[227][228][229] Medications that target this enzyme are undergoing development.[227][228][229] In particular, arachidonate 5-lipoxygenase inhibitors produce massive, rapid programmed cell death in prostate cancer cells.[227][228][229]
Galectin-3 is another potential target.[230] Aberrant glycan profiles have been described in prostate cancer,[231][232] and studies have found specific links between the galectin signature and prostate cancer.[233][234]
The PIM kinase family is another potential target for selective inhibition. A number of related drugs are under development. It has been suggested the most promising approach may be to co-target this family with other pathways including PI3K.[18]
Cancer models[]
Scientists have established prostate cancer cell lines to investigate disease progression. LNCaP, PC-3 (PC3), and DU-145 (DU145) are commonly used prostate cancer cell lines. The LNCaP cancer cell line was established from a human lymph node metastatic lesion of prostatic adenocarcinoma. PC-3 and DU-145 cells were established from human prostatic adenocarcinoma metastatic to bone and to brain, respectively. LNCaP cells express AR, but PC-3 and DU-145 cells express very little or no AR.
The proliferation of LNCaP cells is androgen-dependent but the proliferation of PC-3 and DU-145 cells is androgen-insensitive. Elevation of AR expression is often observed in advanced prostate tumors in patients.[235][236] Some androgen-independent LNCaP sublines have been developed from the ATCC androgen-dependent LNCaP cells after androgen deprivation for study of prostate cancer progression. These androgen-independent LNCaP cells have elevated AR expression and express prostate specific antigen upon androgen treatment. Paradoxically, androgens inhibit the proliferation of these androgen-independent prostate cancer cells.[237][238][239]
Diagnosis[]
One active research area and non-clinically applied investigations involves non-invasive methods of tumor detection. A molecular test that detects the presence of cell-associated PCA3 mRNA in fluid obtained from the prostate and first-void urine sample is under investigation. PCA3 mRNA is expressed almost exclusively by prostate cells and has been shown to be highly over-expressed in prostate cancer cells. The test result is currently reported as a specimen ratio of PCA3 mRNA to PSA mRNA.
The PCA3 test attempts to help decide whether, in men suspected of having prostate cancer (especially if an initial biopsy fails to explain the elevated serum PSA), a biopsy/rebiopsy is needed. The higher the expression of PCA3 in the sample, the greater the likelihood of a positive biopsy.[240] The CDC's Evaluation of Genomic Applications in Practice and Prevention Working Group discourages clinical use.[241]
See also[]
- Prostate Cancer Foundation
- Testicular cancer
References[]
- ^ Jump up to: a b c d e f g h "Prostate Cancer Treatment (PDQ) – Health Professional Version". National Cancer Institute. 2014-04-11. Archived from the original on 5 July 2014. Retrieved 1 July 2014.
- ^ Jump up to: a b c d e f g h i j k "Prostate Cancer Treatment (PDQ) – Patient Version". National Cancer Institute. 2014-04-08. Archived from the original on 5 July 2014. Retrieved 1 July 2014.
- ^ Jump up to: a b c d e f g h i j k l m "Chapter 5.11". World Cancer Report. World Health Organization. 2014. ISBN 978-9283204299.
- ^ Jump up to: a b "SEER Stat Fact Sheets: Prostate Cancer". NCI. Archived from the original on 6 July 2014. Retrieved 18 June 2014.
- ^ Jump up to: a b c Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (November 2018). "Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries". CA: A Cancer Journal for Clinicians. 68 (6): 394–424. doi:10.3322/caac.21492. PMID 30207593. S2CID 52188256.
- ^ "Prostate Cancer". National Cancer Institute. January 1980. Archived from the original on 12 October 2014. Retrieved 12 October 2014.
- ^ Ruddon RW (2007). Cancer biology (4th ed.). Oxford: Oxford University Press. p. 223. ISBN 978-0195175431. Archived from the original on 2015-09-15.
- ^ Koh KA, Sesso HD, Paffenbarger RS, Lee IM (December 2006). "Dairy products, calcium and prostate cancer risk". British Journal of Cancer. 95 (11): 1582–5. doi:10.1038/sj.bjc.6603475. PMC 2360740. PMID 17106437.
- ^ Jump up to: a b Caini S, Gandini S, Dudas M, Bremer V, Severi E, Gherasim A (August 2014). "Sexually transmitted infections and prostate cancer risk: a systematic review and meta-analysis". Cancer Epidemiology. 38 (4): 329–38. doi:10.1016/j.canep.2014.06.002. PMID 24986642.
- ^ Lee MV, Katabathina VS, Bowerson ML, Mityul MI, Shetty AS, Elsayes KM, et al. (2016). "BRCA-associated Cancers: Role of Imaging in Screening, Diagnosis, and Management". Radiographics. 37 (4): 1005–1023. doi:10.1148/rg.2017160144. PMID 28548905.
- ^ "Prostate Cancer Treatment". National Cancer Institute. 6 February 2018. Retrieved 1 March 2018.
Controversy exists regarding the value of screening... reported no clear evidence that screening for prostate cancer decreases the risk of death from prostate cancer
- ^ Jump up to: a b Catalona WJ (March 2018). "Prostate Cancer Screening". The Medical Clinics of North America. 102 (2): 199–214. doi:10.1016/j.mcna.2017.11.001. PMC 5935113. PMID 29406053.
- ^ Jump up to: a b "PSA testing". nhs.uk. 3 January 2015. Retrieved 5 March 2018.
- ^ Jump up to: a b "Final Recommendation Statement: Prostate Cancer: Screening". www.uspreventiveservicestaskforce.org. US Preventive Services Task Force (USPSTF). Retrieved 30 August 2018.
- ^ Grossman DC, Curry SJ, Owens DK, Bibbins-Domingo K, Caughey AB, Davidson KW, et al. (May 2018). "Screening for Prostate Cancer: US Preventive Services Task Force Recommendation Statement". JAMA. 319 (18): 1901–1913. doi:10.1001/jama.2018.3710. PMID 29801017.
- ^ Cabarkapa S, Perera M, McGrath S, Lawrentschuk N (December 2016). "Prostate cancer screening with prostate-specific antigen: A guide to the guidelines". Prostate International. 4 (4): 125–129. doi:10.1016/j.prnil.2016.09.002. PMC 5153437. PMID 27995110.
- ^ Jump up to: a b Stratton J, Godwin M (June 2011). "The effect of supplemental vitamins and minerals on the development of prostate cancer: a systematic review and meta-analysis". Family Practice. 28 (3): 243–52. doi:10.1093/fampra/cmq115. PMID 21273283.
- ^ Jump up to: a b c Luszczak S, Kumar C, Sathyadevan VK, Simpson BS, Gately KA, Whitaker HC, Heavey S (2020). "PIM kinase inhibition: co-targeted therapeutic approaches in prostate cancer". Signal Transduction and Targeted Therapy. 5 (1): 7. doi:10.1038/s41392-020-0109-y. PMC 6992635. PMID 32296034.
- ^ "Chapter 1.1". World Cancer Report. World Health Organization. 2014. ISBN 978-9283204299.
- ^ Jump up to: a b Baade PD, Youlden DR, Krnjacki LJ (February 2009). "International epidemiology of prostate cancer: geographical distribution and secular trends". Molecular Nutrition & Food Research. 53 (2): 171–84. doi:10.1002/mnfr.200700511. PMID 19101947.
- ^ Leslie SW, Soon-Sutton TL, Sajjad H, Siref LE. Prostate Cancer. 2020 Oct 28. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; PMID 29261872.
- ^ Jump up to: a b Miller DC, Hafez KS, Stewart A, Montie JE, Wei JT (September 2003). "Prostate carcinoma presentation, diagnosis, and staging: an update form the National Cancer Data Base" (PDF). Cancer. 98 (6): 1169–78. doi:10.1002/cncr.11635. hdl:2027.42/34379. PMID 12973840. S2CID 22077473.
- ^ van der Cruijsen-Koeter IW, Vis AN, Roobol MJ, Wildhagen MF, de Koning HJ, van der Kwast TH, Schröder FH (July 2005). "Comparison of screen detected and clinically diagnosed prostate cancer in the European randomized study of screening for prostate cancer, section rotterdam". The Journal of Urology. 174 (1): 121–5. doi:10.1097/01.ju.0000162061.40533.0f. PMID 15947595.
- ^ Jump up to: a b Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ (April 2003). "Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults". The New England Journal of Medicine. 348 (17): 1625–38. doi:10.1056/NEJMoa021423. PMID 12711737. S2CID 22714795.
- ^ Hankey BF, Feuer EJ, Clegg LX, Hayes RB, Legler JM, Prorok PC, et al. (June 1999). "Cancer surveillance series: interpreting trends in prostate cancer--part I: Evidence of the effects of screening in recent prostate cancer incidence, mortality, and survival rates". Journal of the National Cancer Institute. 91 (12): 1017–24. doi:10.1093/jnci/91.12.1017. PMID 10379964.
- ^ Breslow N, Chan CW, Dhom G, Drury RA, Franks LM, Gellei B, et al. (November 1977). "Latent carcinoma of prostate at autopsy in seven areas. The International Agency for Research on Cancer, Lyons, France". International Journal of Cancer. 20 (5): 680–8. doi:10.1002/ijc.2910200506. PMID 924691. S2CID 42501757.
- ^ Bell KJ, Del Mar C, Wright G, Dickinson J, Glasziou P (October 2015). "Prevalence of incidental prostate cancer: A systematic review of autopsy studies". International Journal of Cancer. 137 (7): 1749–57. doi:10.1002/ijc.29538. PMC 4682465. PMID 25821151.
- ^ Jahn JL, Giovannucci EL, Stampfer MJ (December 2015). "The high prevalence of undiagnosed prostate cancer at autopsy: implications for epidemiology and treatment of prostate cancer in the Prostate-specific Antigen-era". International Journal of Cancer. 137 (12): 2795–802. doi:10.1002/ijc.29408. PMC 4485977. PMID 25557753.
- ^ Martin RM, Vatten L, Gunnell D, Romundstad P (March 2010). "Blood pressure and risk of prostate cancer: Cohort Norway (CONOR)". Cancer Causes & Control. 21 (3): 463–72. doi:10.1007/s10552-009-9477-x. PMID 19949849. S2CID 30484327.
- ^ Friedenreich CM, Neilson HK, Lynch BM (September 2010). "State of the epidemiological evidence on physical activity and cancer prevention". European Journal of Cancer. 46 (14): 2593–604. doi:10.1016/j.ejca.2010.07.028. PMID 20843488.
- ^ Gann PH, Hennekens CH, Ma J, Longcope C, Stampfer MJ (August 1996). "Prospective study of sex hormone levels and risk of prostate cancer". Journal of the National Cancer Institute. 88 (16): 1118–26. doi:10.1093/jnci/88.16.1118. PMID 8757191.
- ^ "Prostate cancer". Genetics Home Reference. Retrieved 1 May 2020.
- ^ Steinberg GD, Carter BS, Beaty TH, Childs B, Walsh PC (1990). "Family history and the risk of prostate cancer". The Prostate. 17 (4): 337–47. doi:10.1002/pros.2990170409. PMID 2251225. S2CID 44925478.
- ^ Zeegers MP, Jellema A, Ostrer H (April 2003). "Empiric risk of prostate carcinoma for relatives of patients with prostate carcinoma: a meta-analysis". Cancer. 97 (8): 1894–903. doi:10.1002/cncr.11262. PMID 12673715. S2CID 12607885.
- ^ Jump up to: a b Gallagher RP, Fleshner N (October 1998). "Prostate cancer: 3. Individual risk factors" (PDF). CMAJ. 159 (7): 807–13. PMC 1232741. PMID 9805030. Archived (PDF) from the original on 2009-12-29.
- ^ Hoffman RM, Gilliland FD, Eley JW, Harlan LC, Stephenson RA, Stanford JL, et al. (March 2001). "Racial and ethnic differences in advanced-stage prostate cancer: the Prostate Cancer Outcomes Study". Journal of the National Cancer Institute. 93 (5): 388–95. doi:10.1093/jnci/93.5.388. PMID 11238701.
- ^ Lichtenstein P, Holm NV, Verkasalo PK, Iliadou A, Kaprio J, Koskenvuo M, et al. (July 2000). "Environmental and heritable factors in the causation of cancer--analyses of cohorts of twins from Sweden, Denmark, and Finland". The New England Journal of Medicine. 343 (2): 78–85. doi:10.1056/NEJM200007133430201. PMID 10891514.
- ^ Struewing JP, Hartge P, Wacholder S, Baker SM, Berlin M, McAdams M, et al. (May 1997). "The risk of cancer associated with specific mutations of BRCA1 and BRCA2 among Ashkenazi Jews". The New England Journal of Medicine. 336 (20): 1401–8. doi:10.1056/NEJM199705153362001. PMID 9145676.
- ^ Beuzeboc P, Soulié M, Richaud P, Salomon L, Staerman F, Peyromaure M, et al. (December 2009). "[Fusion genes and prostate cancer. From discovery to prognosis and therapeutic perspectives]". Progres en Urologie (in French). 19 (11): 819–24. doi:10.1016/j.purol.2009.06.002. PMID 19945666.
- ^ Baca SC, Prandi D, Lawrence MS, Mosquera JM, Romanel A, Drier Y, et al. (April 2013). "Punctuated evolution of prostate cancer genomes". Cell. 153 (3): 666–77. doi:10.1016/j.cell.2013.03.021. PMC 3690918. PMID 23622249.
- ^ Eeles RA, Kote-Jarai Z, Giles GG, Olama AA, Guy M, Jugurnauth SK, et al. (March 2008). "Multiple newly identified loci associated with prostate cancer susceptibility". Nature Genetics. 40 (3): 316–21. doi:10.1038/ng.90. PMID 18264097. S2CID 30968525.
- ^ Thomas G, Jacobs KB, Yeager M, Kraft P, Wacholder S, Orr N, et al. (March 2008). "Multiple loci identified in a genome-wide association study of prostate cancer". Nature Genetics. 40 (3): 310–5. doi:10.1038/ng.91. PMID 18264096. S2CID 22978381.
- ^ Whitaker HC, Kote-Jarai Z, Ross-Adams H, Warren AY, Burge J, George A, et al. (October 2010). Vickers A (ed.). "The rs10993994 risk allele for prostate cancer results in clinically relevant changes in microseminoprotein-beta expression in tissue and urine". PLOS ONE. 5 (10): e13363. Bibcode:2010PLoSO...513363W. doi:10.1371/journal.pone.0013363. PMC 2954177. PMID 20967219.
- ^ Berndt SI, Wang Z, Yeager M, Alavanja MC, Albanes D, Amundadottir L, et al. (May 2015). "Two susceptibility loci identified for prostate cancer aggressiveness". Nature Communications. 6: 6889. Bibcode:2015NatCo...6.6889.. doi:10.1038/ncomms7889. PMC 4422072. PMID 25939597.
- ^ Venkateswaran V, Klotz LH (August 2010). "Diet and prostate cancer: mechanisms of action and implications for chemoprevention". Nature Reviews. Urology. 7 (8): 442–53. doi:10.1038/nrurol.2010.102. PMID 20647991. S2CID 10602814.
- ^ "Chemicals in Meat Cooked at High Temperatures and Cancer Risk". National Cancer Institute. 2018-04-02. Archived from the original on 2011-11-06.
- ^ "Milk and Health". American College of Cardiology. Retrieved 2021-02-21.
- ^ Willett WC, Ludwig DS (February 2020). "Milk and Health". The New England Journal of Medicine. 382 (7): 644–654. doi:10.1056/NEJMra1903547. PMID 32053300.
- ^ Sargsyan A, Dubasi HB (July 2020). "Milk Consumption and Prostate Cancer: A Systematic Review". The World Journal of Men's Health. 38 (3): 419–428. doi:10.5534/wjmh.200051. PMC 8255404. PMID 32777868.
- ^ López-Plaza B, Bermejo LM, Santurino C, Cavero-Redondo I, Álvarez-Bueno C, Gómez-Candela C (May 2019). "Milk and dairy product consumption and prostate cancer risk and mortality: an overview of systematic reviews and meta-analyses". Adv Nutr. 10 (suppl_2): S212–S223. doi:10.1093/advances/nmz014. PMC 6518142. PMID 31089741.
- ^ Wigle DT, Turner MC, Gomes J, Parent ME (March 2008). "Role of hormonal and other factors in human prostate cancer". Journal of Toxicology and Environmental Health Part B: Critical Reviews. 11 (3–4): 242–59. doi:10.1080/10937400701873548. PMID 18368555. S2CID 24489849.
- ^ Qin X, Cui Y, Shen L, Sun N, Zhang Y, Li J, et al. (September 2013). "Folic acid supplementation and cancer risk: a meta-analysis of randomized controlled trials". International Journal of Cancer. 133 (5): 1033–41. doi:10.1002/ijc.28038. PMID 23338728. S2CID 19830376.
- ^ Jacobs EJ, Rodriguez C, Mondul AM, Connell CJ, Henley SJ, Calle EE, Thun MJ (July 2005). "A large cohort study of aspirin and other nonsteroidal anti-inflammatory drugs and prostate cancer incidence". Journal of the National Cancer Institute. 97 (13): 975–80. doi:10.1093/jnci/dji173. PMID 15998950.
- ^ Shannon J, Tewoderos S, Garzotto M, Beer TM, Derenick R, Palma A, Farris PE (August 2005). "Statins and prostate cancer risk: a case-control study". American Journal of Epidemiology. 162 (4): 318–25. doi:10.1093/aje/kwi203. PMID 16014776.
- ^ Dennis LK, Lynch CF, Torner JC (July 2002). "Epidemiologic association between prostatitis and prostate cancer". Urology. 60 (1): 78–83. doi:10.1016/S0090-4295(02)01637-0. PMID 12100928.
- ^ Heidegger I, Borena W, Pichler R (May 2015). "The role of human papilloma virus in urological malignancies". Anticancer Research. 35 (5): 2513–9. PMID 25964524.
- ^ Cai T, Di Vico T, Durante J, Tognarelli A, Bartoletti R (December 2018). "Human papilloma virus and genitourinary cancers: a narrative review". Minerva Urologica e Nefrologica. 70 (6): 579–587. doi:10.23736/S0393-2249.18.03141-7. PMID 30160386.
- ^ Rider JR, Wilson KM, Sinnott JA, Kelly RS, Mucci LA, Giovannucci EL (December 2016). "Ejaculation Frequency and Risk of Prostate Cancer: Updated Results with an Additional Decade of Follow-up". European Urology. 70 (6): 974–982. doi:10.1016/j.eururo.2016.03.027. PMC 5040619. PMID 27033442.
- ^ Aboul-Enein BH, Bernstein J, Ross MW (July 2016). "Evidence for Masturbation and Prostate Cancer Risk: Do We Have a Verdict?". Sexual Medicine Reviews. 4 (3): 229–234. doi:10.1016/j.sxmr.2016.02.006. PMID 27871956.
- ^ "A comprehensive cancer control program for BC". Archived from the original on 27 September 2006. Retrieved 9 August 2010.
- ^ Aumüller G (1979). Prostate Gland and Seminal Vesicles. Berlin-Heidelberg: Springer-Verlag.
- ^ Moore KL, Chubb D (1999). Clinically Oriented Anatomy. Baltimore, Maryland: Lippincott Williams & Wilkins. ISBN 978-0-683-06132-1.
- ^ Steive H (1930). "Männliche Genitalorgane". Handbuch der mikroskopischen Anatomie des Menschen. Vol. VII Part 2. Berlin: Springer. pp. 1–399.
- ^ McNeal JE (1984). "Anatomy of the prostate and morphogenesis of BPH". Progress in Clinical and Biological Research. 145: 27–53. PMID 6201879.
- ^ Oh WK, Hurwitz M, D'Amico AV, Richie JP, Kantoff PW (2003). "Biology of Prostate Cancer". Holland-Frei Cancer Medicine (6th ed.).
- ^ Reissigl A, Pointner J, Strasser H, Ennemoser O, Klocker H, Bartsch G (February 1997). "Frequency and clinical significance of transition zone cancer in prostate cancer screening". The Prostate. 30 (2): 130–5. doi:10.1002/(SICI)1097-0045(19970201)30:2<130::AID-PROS8>3.0.CO;2-S. PMID 9051151.
- ^ "Male Genitals – Prostate Neoplasms". Pathology study images. University of Virginia School of Medicine. Archived from the original on 2011-07-20. Retrieved 2011-04-28.
There are many connections between the prostatic venous plexus and the vertebral veins. The veins forming the prostatic plexus do not contain valves and it is thought that straining to urinate causes prostatic venous blood to flow in a reverse direction and enter the vertebral veins carrying malignant cells to the vertebral column.
- ^ Costello LC, Franklin RB (May 2006). "The clinical relevance of the metabolism of prostate cancer; zinc and tumor suppression: connecting the dots". Molecular Cancer. 5: 17. doi:10.1186/1476-4598-5-17. PMC 1481516. PMID 16700911.
- ^ "Scientists Discover Anti-Cancer Mechanism that Arrests Early Prostate Cancer". August 4, 2005. Archived from the original on May 19, 2008.
- ^ Sharma A, Yeow WS, Ertel A, Coleman I, Clegg N, Thangavel C, et al. (December 2010). "The retinoblastoma tumor suppressor controls androgen signaling and human prostate cancer progression". The Journal of Clinical Investigation. 120 (12): 4478–92. doi:10.1172/JCI44239. PMC 2993601. PMID 21099110.
- ^ Leav I, Plescia J, Goel HL, Li J, Jiang Z, Cohen RJ, et al. (January 2010). "Cytoprotective mitochondrial chaperone TRAP-1 as a novel molecular target in localized and metastatic prostate cancer". The American Journal of Pathology. 176 (1): 393–401. doi:10.2353/ajpath.2010.090521. PMC 2797899. PMID 19948822.
- ^ Zha J, Huang YF (September 2009). "[TGF-beta/Smad in prostate cancer: an update]". Zhonghua Nan Ke Xue = National Journal of Andrology (in Chinese). 15 (9): 840–3. PMID 19947572.
- ^ Watanabe S, Miyata Y, Kanda S, Iwata T, Hayashi T, Kanetake H, Sakai H (May 2010). "Expression of X-linked inhibitor of apoptosis protein in human prostate cancer specimens with and without neo-adjuvant hormonal therapy". Journal of Cancer Research and Clinical Oncology. 136 (5): 787–93. doi:10.1007/s00432-009-0718-x. PMID 19946707. S2CID 34855148.
- ^ Senapati S, Rachagani S, Chaudhary K, Johansson SL, Singh RK, Batra SK (March 2010). "Overexpression of macrophage inhibitory cytokine-1 induces metastasis of human prostate cancer cells through the FAK-RhoA signaling pathway". Oncogene. 29 (9): 1293–302. doi:10.1038/onc.2009.420. PMC 2896817. PMID 19946339.
- ^ Narizhneva NV, Tararova ND, Ryabokon P, Shyshynova I, Prokvolit A, Komarov PG, et al. (December 2009). "Small molecule screening reveals a transcription-independent pro-survival function of androgen receptor in castration-resistant prostate cancer". Cell Cycle. 8 (24): 4155–67. doi:10.4161/cc.8.24.10316. PMC 2896895. PMID 19946220.
- ^ Yao V, Berkman CE, Choi JK, O'Keefe DS, Bacich DJ (February 2010). "Expression of prostate-specific membrane antigen (PSMA), increases cell folate uptake and proliferation and suggests a novel role for PSMA in the uptake of the non-polyglutamated folate, folic acid". The Prostate. 70 (3): 305–16. doi:10.1002/pros.21065. PMID 19830782. S2CID 21518526.
- ^ "Cancer Screening Guidelines | Detecting Cancer Early". Archived from the original on 2011-06-13. Retrieved 2011-06-16. American Cancer Society American Cancer Society Guidelines for the early detection of cancer Cited: September 2011
- ^ Georgiev, A. (2016). Case of prostate cancer with anterior localization - Multiparametric MRI study. Rentgenologiya i Radiologiya, 55(4), 285–87.
- ^ Jump up to: a b c d e f Drost FH, Osses DF, Nieboer D, Steyerberg EW, Bangma CH, Roobol MJ, Schoots IG, et al. (Cochrane Urology Group) (April 2019). "Prostate MRI, with or without MRI-targeted biopsy, and systematic biopsy for detecting prostate cancer". The Cochrane Database of Systematic Reviews. 4: CD012663. doi:10.1002/14651858.CD012663.pub2. PMC 6483565. PMID 31022301.
- ^ Wang X, Bao J, Ping X, Hu C, Hou J, Dong F, Guo L (September 2018). "The diagnostic value of PI-RADS V1 and V2 using multiparametric MRI in transition zone prostate clinical cancer". Oncology Letters. 16 (3): 3201–3206. doi:10.3892/ol.2018.9038. PMC 6096261. PMID 30127915.
- ^ Tan N, Margolis DJ, McClure TD, Thomas A, Finley DS, Reiter RE, et al. (August 2012). "Radical prostatectomy: value of prostate MRI in surgical planning". Abdominal Imaging. 37 (4): 664–74. doi:10.1007/s00261-011-9805-y. PMID 21993567. S2CID 20471235.
- ^ Scheltema MJ, Tay KJ, Postema AW, de Bruin DM, Feller J, Futterer JJ, et al. (May 2017). "Utilization of multiparametric prostate magnetic resonance imaging in clinical practice and focal therapy: report from a Delphi consensus project". World Journal of Urology. 35 (5): 695–701. doi:10.1007/s00345-016-1932-1. OCLC 1188365278. PMC 5397427. PMID 27637908.
- ^ Wang T, Zhou J, Tian S, Wang Y, Patel P, Jani AB, et al. (March 2020). "A planning study of focal dose escalations to multiparametric MRI-defined dominant intraprostatic lesions in prostate proton radiation therapy". The British Journal of Radiology. 93 (1107): 20190845. doi:10.1259/bjr.20190845. PMC 7066949. PMID 31904261.
- ^ Heavey S, Haider A, Sridhar A, Pye H, Shaw G, Freeman A, Whitaker H (October 2019). "Use of Magnetic Resonance Imaging and Biopsy Data to Guide Sampling Procedures for Prostate Cancer Biobanking". Journal of Visualized Experiments (152). doi:10.3791/60216. PMID 31657791.
- ^ Heavey S, Costa H, Pye H, Burt EC, Jenkinson S, Lewis GR, et al. (May 2019). "PEOPLE: PatiEnt prOstate samPLes for rEsearch, a tissue collection pathway utilizing magnetic resonance imaging data to target tumor and benign tissue in fresh radical prostatectomy specimens". The Prostate. 79 (7): 768–777. doi:10.1002/pros.23782. PMC 6618051. PMID 30807665.
- ^ Jump up to: a b c Norris JM, Simpson BS, Freeman A, Kirkham A, Whitaker HC, Emberton M (November 2020). "Conspicuity of prostate cancer on multiparametric magnetic resonance imaging: A cross-disciplinary translational hypothesis". FASEB Journal. 34 (11): 14150–14159. doi:10.1096/fj.202001466R. PMID 32920937. S2CID 221675029.
- ^ Truong M, Hollenberg G, Weinberg E, Messing EM, Miyamoto H, Frye TP (August 2017). "Impact of Gleason Subtype on Prostate Cancer Detection Using Multiparametric Magnetic Resonance Imaging: Correlation with Final Histopathology". The Journal of Urology. 198 (2): 316–321. doi:10.1016/j.juro.2017.01.077. PMID 28163032. S2CID 45430609.
- ^ Norris JM, Carmona Echeverria LM, Simpson BS, Allen C, Ball R, Freeman A, et al. (August 2020). "Prostate cancer visibility on multiparametric magnetic resonance imaging: high Gleason grade and increased tumour volume are not the only important histopathological features". BJU International. 126 (2): 237–239. doi:10.1111/bju.15085. PMID 32319152.
- ^ Jump up to: a b Norris JM, Simpson BS, Parry MA, Allen C, Ball R, Freeman A, et al. (July 2020). "Genetic Landscape of Prostate Cancer Conspicuity on Multiparametric Magnetic Resonance Imaging: A Systematic Review and Bioinformatic Analysis". European Urology Open Science. 20: 37–47. doi:10.1016/j.euros.2020.06.006. PMC 7497895. PMID 33000006.
- ^ Jump up to: a b Drost, Frank-Jan H.; Osses, Daniel; Nieboer, Daan; Bangma, Chris H.; Steyerberg, Ewout W.; Roobol, Monique J.; Schoots, Ivo G. (January 2020). "Prostate Magnetic Resonance Imaging, with or Without Magnetic Resonance Imaging-targeted Biopsy, and Systematic Biopsy for Detecting Prostate Cancer: A Cochrane Systematic Review and Meta-analysis". European Urology. 77 (1): 78–94. doi:10.1016/j.eururo.2019.06.023. PMID 31326219.
- ^ Yaghi MD, Kehinde EO (2015). "Oral antibiotics in trans-rectal prostate biopsy and its efficacy to reduce infectious complications: Systematic review". Urology Annals. 7 (4): 417–27. doi:10.4103/0974-7796.164860. PMC 4660689. PMID 26538868.
- ^ Zani EL, Clark OA, Rodrigues Netto N (May 2011). "Antibiotic prophylaxis for transrectal prostate biopsy". The Cochrane Database of Systematic Reviews (5): CD006576. doi:10.1002/14651858.CD006576.pub2. PMID 21563156.
- ^ Essink-Bot ML, de Koning HJ, Nijs HG, Kirkels WJ, van der Maas PJ, Schröder FH (June 1998). "Short-term effects of population-based screening for prostate cancer on health-related quality of life". Journal of the National Cancer Institute. 90 (12): 925–31. doi:10.1093/jnci/90.12.925. PMID 9637143.
- ^ References for pie chart are located in table in the article Histopathologic diagnosis of prostate cancer: Incidences generally include cases where the pattern is found admixed with usual acinar adenocarcinoma.
- ^ Li J, Wang Z (February 2016). "The pathology of unusual subtypes of prostate cancer". Chinese Journal of Cancer Research = Chung-Kuo Yen Cheng Yen Chiu. 28 (1): 130–43. doi:10.3978/j.issn.1000-9604.2016.01.06. PMC 4779761. PMID 27041935.
- ^ Baig FA, Hamid A, Mirza T, Syed S (May 2015). "Ductal and Acinar Adenocarcinoma of Prostate: Morphological and Immunohistochemical Characterization". Oman Medical Journal. 30 (3): 162–6. doi:10.5001/omj.2015.36. PMC 4459157. PMID 26171121.
- ^ "Prostatic Adenocarcinoma". Stanford University School of Medicine. Retrieved 2019-10-30.
- ^ Jump up to: a b Rao SR, Snaith AE, Marino D, Cheng X, Lwin ST, Orriss IR, et al. (January 2017). "Tumour-derived alkaline phosphatase regulates tumour growth, epithelial plasticity and disease-free survival in metastatic prostate cancer". British Journal of Cancer. 116 (2): 227–236. doi:10.1038/bjc.2016.402. PMC 5243990. PMID 28006818.
- ^ "Male Genital Pathology". Retrieved 2009-05-13.
- ^ Chuang AY, DeMarzo AM, Veltri RW, Sharma RB, Bieberich CJ, Epstein JI (August 2007). "Immunohistochemical differentiation of high-grade prostate carcinoma from urothelial carcinoma". The American Journal of Surgical Pathology. 31 (8): 1246–55. doi:10.1097/PAS.0b013e31802f5d33. PMID 17667550. S2CID 11535862.
- ^ Jump up to: a b Nutting C, Horwich A, Fisher C, Parsons C, Dearnaley DP (June 1997). "Small-cell carcinoma of the prostate". Journal of the Royal Society of Medicine. 90 (6): 340–1. doi:10.1177/014107689709000615. PMC 1296316. PMID 9227387.
- ^ Jump up to: a b Wei ZF, Xu H, Wang H, Wei W, Cheng W, Zhou WQ, et al. (September 2009). "[Clinicopathological characterization of prostatic small cell carcinoma: a case report and review of the literature]". Zhonghua Nan Ke Xue = National Journal of Andrology (in Chinese). 15 (9): 829–32. PMID 19947569.
- ^ Catz SD, Johnson JL (January 2003). "BCL-2 in prostate cancer: a minireview". Apoptosis. 8 (1): 29–37. doi:10.1023/A:1021692801278. PMID 12510149. S2CID 21948907.
- ^ BMJ Group (8 December 2009). "Prostate cancer: How far has your cancer spread? The TNM system". Guardian.co.uk. London. Archived from the original on 4 April 2009. Retrieved 9 August 2010.
- ^ American Society of Clinical Oncology (April 2013). "Five things physicians and patients should question" (PDF). The Journal of the Oklahoma State Medical Association. 106 (4): 150–1. PMID 23795527. Archived from the original (PDF) on 2012-07-31.
- Makarov DV, Desai RA, Yu JB, Sharma R, Abraham N, Albertsen PC, et al. (January 2012). "The population level prevalence and correlates of appropriate and inappropriate imaging to stage incident prostate cancer in the medicare population". The Journal of Urology. 187 (1): 97–102. doi:10.1016/j.juro.2011.09.042. PMID 22088337.
- National Comprehensive Cancer Network – Prostate (2012). "NCCN Clinical Practice Guidelines in Oncology". nccn.org. Archived from the original on 9 November 2012. Retrieved 15 November 2012.
- Thompson I, Thrasher JB, Aus G, Burnett AL, Canby-Hagino ED, Cookson MS, et al. (June 2007). "Guideline for the management of clinically localized prostate cancer: 2007 update". The Journal of Urology. 177 (6): 2106–31. doi:10.1016/j.juro.2007.03.003. PMID 17509297.
- ^ Hofman MS, Lawrentschuk N, Francis RJ, Tang C, Vela I, Thomas P, et al. (April 2020). "Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): a prospective, randomised, multicentre study" (PDF). Lancet. 395 (10231): 1208–1216. doi:10.1016/S0140-6736(20)30314-7. PMID 32209449. S2CID 214609500.
- ^ Jump up to: a b c d e Masko EM, Allott EH, Freedland SJ (May 2013). "The relationship between nutrition and prostate cancer: is more always better?". European Urology. 63 (5): 810–20. doi:10.1016/j.eururo.2012.11.012. PMC 3597758. PMID 23219353.
- ^ Thompson AK, Shaw DI, Minihane AM, Williams CM (December 2008). "Trans-fatty acids and cancer: the evidence reviewed". Nutrition Research Reviews. 21 (2): 174–88. doi:10.1017/S0954422408110964. PMID 19087370.
- ^ Heinze VM, Actis AB (February 2012). "Dietary conjugated linoleic acid and long-chain n-3 fatty acids in mammary and prostate cancer protection: a review". International Journal of Food Sciences and Nutrition. 63 (1): 66–78. doi:10.3109/09637486.2011.598849. PMID 21762028. S2CID 21614046.
- ^ Datta M, Schwartz GG (2012). "Calcium and vitamin D supplementation during androgen deprivation therapy for prostate cancer: a critical review". The Oncologist. 17 (9): 1171–9. doi:10.1634/theoncologist.2012-0051. PMC 3448410. PMID 22836449.
- ^ Szymanski KM, Wheeler DC, Mucci LA (November 2010). "Fish consumption and prostate cancer risk: a review and meta-analysis". The American Journal of Clinical Nutrition. 92 (5): 1223–33. doi:10.3945/ajcn.2010.29530. PMID 20844069.
- ^ American Dietetic Association (June 2003). "Position of the American Dietetic Association and Dietitians of Canada: Vegetarian diets". Journal of the American Dietetic Association. 103 (6): 748–65. doi:10.1053/jada.2003.50142. PMID 12778049.
- ^ World Cancer Research Fund; American Institute for Cancer (2007). Food, nutrition, physical activity, and the prevention of cancer a global perspective (PDF). Washington, D.C.: American Institute for Cancer Research. p. 76. ISBN 978-0-9722522-2-5. Archived from the original (PDF) on 2013-05-23.CS1 maint: multiple names: authors list (link)
- ^ Rowles JL, Ranard KM, Applegate CC, Jeon S, An R, Erdman JW (September 2018). "Processed and raw tomato consumption and risk of prostate cancer: a systematic review and dose-response meta-analysis". Prostate Cancer and Prostatic Diseases. 21 (3): 319–336. doi:10.1038/s41391-017-0005-x. PMID 29317772. S2CID 3306182.
- ^ Jump up to: a b "American Cancer Society Guidelines on Nutrition and Physical Activity for Cancer Prevention" (PDF). American Cancer Society. Archived (PDF) from the original on 2012-06-25.
- ^ Wilt TJ, MacDonald R, Hagerty K, Schellhammer P, Kramer BS (April 2008). Wilt TJ (ed.). "Five-alpha-reductase Inhibitors for prostate cancer prevention". The Cochrane Database of Systematic Reviews (2): CD007091. doi:10.1002/14651858.CD007091. PMID 18425978.
- ^ Jump up to: a b Alberts AR, Schoots IG, Roobol MJ (June 2015). "Prostate-specific antigen-based prostate cancer screening: Past and future". International Journal of Urology. 22 (6): 524–32. doi:10.1111/iju.12750. PMID 25847604. S2CID 7525080.
- ^ Jump up to: a b Rendon RA, Mason RJ, Marzouk K, Finelli A, Saad F, So A, et al. (October 2017). "Canadian Urological Association recommendations on prostate cancer screening and early diagnosis". Canadian Urological Association Journal = Journal de l'Association des Urologues du Canada. 11 (10): 298–309. doi:10.5489/cuaj.4888. PMC 5659858. PMID 29381452.
- ^ "Home Page – USPSTF Draft Prostate Screening Recommendations". USPSTF Draft Prostate Screening Recommendations. Archived from the original on 2 March 2018. Retrieved 2 March 2018.
- ^ Mulhem E, Fulbright N, Duncan N (October 2015). "Prostate Cancer Screening". American Family Physician. 92 (8): 683–8. PMID 26554408.
- ^ Prostate Cancer Screening Archived 2017-09-07 at the Wayback Machine CDC, updated April 6, 2010
- ^ Qaseem A, Barry MJ, Denberg TD, Owens DK, Shekelle P (May 2013). "Screening for prostate cancer: a guidance statement from the Clinical Guidelines Committee of the American College of Physicians". Annals of Internal Medicine. 158 (10): 761–769. doi:10.7326/0003-4819-158-10-201305210-00633. PMID 23567643.
- ^ Basch E, Oliver TK, Vickers A, Thompson I, Kantoff P, Parnes H, et al. (August 2012). "Screening for prostate cancer with prostate-specific antigen testing: American Society of Clinical Oncology Provisional Clinical Opinion". Journal of Clinical Oncology. 30 (24): 3020–5. doi:10.1200/JCO.2012.43.3441. PMC 3776923. PMID 22802323.
- ^ "Early Detection of Prostate Cancer: AUA Guidelines". American Urological Association. 2013. Archived from the original on 7 May 2013. Retrieved 10 May 2013.
- ^ Jump up to: a b c d Filson CP, Marks LS, Litwin MS (8 May 2015). "Expectant management for men with early stage prostate cancer". Ca. 65 (4): 265–82. doi:10.3322/caac.21278. PMID 25958817. S2CID 36057004.
- ^ Jayadevappa R, Chhatre S, Wong YN, Wittink MN, Cook R, Morales KH, et al. (May 2017). "Comparative effectiveness of prostate cancer treatments for patient-centered outcomes: A systematic review and meta-analysis (PRISMA Compliant)". Medicine. 96 (18): e6790. doi:10.1097/MD.0000000000006790. PMC 5419922. PMID 28471976.
- ^ Lu-Yao GL, Albertsen PC, Moore DF, Shih W, Lin Y, DiPaola RS, et al. (September 2009). "Outcomes of localized prostate cancer following conservative management". JAMA. 302 (11): 1202–9. doi:10.1001/jama.2009.1348. PMC 2822438. PMID 19755699.
- ^ Mongiat-Artus P, Peyromaure M, Richaud P, Droz JP, Rainfray M, Jeandel C, et al. (December 2009). "[Recommendations for the treatment of prostate cancer in the elderly man: A study by the oncology committee of the French association of urology]". Progres en Urologie (in French). 19 (11): 810–7. doi:10.1016/j.purol.2009.02.008. PMID 19945664.
- ^ Picard JC, Golshayan AR, Marshall DT, Opfermann KJ, Keane TE (November 2009). "The multi-disciplinary management of high-risk prostate cancer". Urologic Oncology. 30 (1): 3–15. doi:10.1016/j.urolonc.2009.09.002. PMID 19945310.
- ^ Fitzpatrick JM (March 2008). "Management of localized prostate cancer in senior adults: the crucial role of comorbidity". BJU International. 101 Suppl 2 (Suppl 2): 16–22. doi:10.1111/j.1464-410X.2007.07487.x. PMID 18307688. S2CID 205538470.
- ^ "Evidence-Based Cancer Guidelines, Oncology Drug Compendium, Oncology Continuing Medical Education". NCCN. Retrieved 2011-08-29.
- ^ Jump up to: a b Mohan R, Schellhammer PF (August 2011). "Treatment options for localized prostate cancer". American Family Physician. 84 (4): 413–20. PMID 21842788.
- ^ "Active Surveillance for the Management of Localized Prostate Cancer". Cancer Care Ontario. Archived from the original on 2020-04-10.
- ^ "Active Surveillance May Be Preferred Option in Some Men with Prostate Cancer". Cancer.gov. 2011-04-19. Archived from the original on 2011-05-03. Retrieved 2011-08-29.
- ^ Jump up to: a b c d e Vernooij, Robin Wm; Lancee, Michelle; Cleves, Anne; Dahm, Philipp; Bangma, Chris H.; Aben, Katja Kh (June 4, 2020). "Radical prostatectomy versus deferred treatment for localised prostate cancer". The Cochrane Database of Systematic Reviews. 6: CD006590. doi:10.1002/14651858.CD006590.pub3. ISSN 1469-493X. PMC 7270852. PMID 32495338.
- ^ Sartor O, de Bono JS (February 2018). "Metastatic Prostate Cancer". The New England Journal of Medicine. 378 (7): 645–657. doi:10.1056/NEJMra1701695. PMID 29412780.
- ^ Dhondt B, De Bleser E, Claeys T, Buelens S, Lumen N, Vandesompele J, et al. (December 2019). "Discovery and validation of a serum microRNA signature to characterize oligo- and polymetastatic prostate cancer: not ready for prime time". World Journal of Urology. 37 (12): 2557–2564. doi:10.1007/s00345-018-2609-8. PMID 30578441. S2CID 58594673.
- ^ Hammerstrom AE, Cauley DH, Atkinson BJ, Sharma P (August 2011). "Cancer immunotherapy: sipuleucel-T and beyond". Pharmacotherapy. 31 (8): 813–28. doi:10.1592/phco.31.8.813. PMC 4159742. PMID 21923608.
- ^ "Prostate Cancer: Radical Prostatectomy". WebMD. Retrieved 10 May 2020.
- ^ Mouraviev V, Evans B, Polascik TJ (2006). "Salvage prostate cryoablation after primary interstitial brachytherapy failure: a feasible approach". Prostate Cancer and Prostatic Diseases. 9 (1): 99–101. doi:10.1038/sj.pcan.4500853. PMID 16314889.
- ^ Wallis CJ, Mahar AL, Choo R, Herschorn S, Kodama RT, Shah PS, et al. (March 2016). "Second malignancies after radiotherapy for prostate cancer: systematic review and meta-analysis". BMJ. 352: i851. doi:10.1136/bmj.i851. PMC 4775870. PMID 26936410.
- ^ Wallis CJ, Glaser A, Hu JC, Huland H, Lawrentschuk N, Moon D, et al. (January 2018). "Survival and Complications Following Surgery and Radiation for Localized Prostate Cancer: An International Collaborative Review" (PDF). European Urology. 73 (1): 11–20. doi:10.1016/j.eururo.2017.05.055. PMID 28610779.
- ^ Hong H, Zhang Y, Sun J, Cai W (June 2010). "Positron emission tomography imaging of prostate cancer". Amino Acids. 39 (1): 11–27. doi:10.1007/s00726-009-0394-9. PMC 2883014. PMID 19946787.
- ^ Peyromaure M, Valéri A, Rebillard X, Beuzeboc P, Richaud P, Soulié M, Salomon L (December 2009). "[Characteristics of prostate cancer in men less than 50-year-old]". Progres en Urologie (in French). 19 (11): 803–9. doi:10.1016/j.purol.2009.04.010. PMID 19945663.
- ^ "Castrate-resistant prostate cancer: In NCI Dictionary of Cancer Terms". National Cancer Institute, US National Institutes of Health. 2019. Retrieved 17 September 2019.
- ^ "Treatments for castrate-resistant prostate cancer". Canadian Cancer Society. 2019. Retrieved 17 September 2019.
- ^ Seruga B, Ocana A, Tannock IF (January 2011). "Drug resistance in metastatic castration-resistant prostate cancer". Nature Reviews. Clinical Oncology. 8 (1): 12–23. doi:10.1038/nrclinonc.2010.136. PMID 20859283. S2CID 24512148.
- ^ Clarke NW (2005). "Docetaxel for the Treatment of Hormone Refractory Prostate Cancer" (PDF). Archived from the original (PDF) on 2012-07-12.
- ^ "Prostate cancer (hormone-refractory) - docetaxel". National Institute for Health and Clinical Excellence. 2010-12-10. Archived from the original on 2012-02-02. Retrieved 2011-07-04.
- ^ de Bono JS, Oudard S, Ozguroglu M, Hansen S, Machiels JP, Kocak I, et al. (October 2010). "Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial". Lancet. 376 (9747): 1147–54. doi:10.1016/S0140-6736(10)61389-X. PMID 20888992. S2CID 4791847.
- ^ "Avastin, Thalomid, Taxotere, and Prednisone Effective for Men with Hormone Refractory Prostate Cancer". March 2010. Archived from the original on 15 June 2010. Retrieved 10 May 2010.
- ^ Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, et al. (July 2010). "Sipuleucel-T immunotherapy for castration-resistant prostate cancer". The New England Journal of Medicine. 363 (5): 411–22. doi:10.1056/NEJMoa1001294. PMID 20818862. S2CID 12168204.
- ^ "FDA approves Zytiga for late-stage prostate cancer". U.S. Food and Drug Administration. 2011-04-28. Archived from the original on 2013-09-22.
- ^ Jump up to: a b c "FDA approves new treatment for a type of late stage prostate cancer" (Press release). Food and Drug Administration. 2012-08-31. Archived from the original on 2013-10-02.
- ^ Jump up to: a b c Azvolinsky A (September 4, 2012). "FDA Approves Enzalutamide (Xtandi) for Late-Stage Prostate Cancer". CancerNetwork. Archived from the original on September 13, 2012.
- ^ Qin J, Liu X, Laffin B, Chen X, Choy G, Jeter CR, et al. (May 2012). "The PSA(-/lo) prostate cancer cell population harbors self-renewing long-term tumor-propagating cells that resist castration". Cell Stem Cell. 10 (5): 556–69. doi:10.1016/j.stem.2012.03.009. PMC 3348510. PMID 22560078.
- ^ Maitland NJ, Collins AT (June 2008). "Prostate cancer stem cells: a new target for therapy". Journal of Clinical Oncology. 26 (17): 2862–70. doi:10.1200/JCO.2007.15.1472. PMID 18539965.
- ^ Attard G, Richards J, de Bono JS (April 2011). "New strategies in metastatic prostate cancer: targeting the androgen receptor signaling pathway". Clinical Cancer Research. 17 (7): 1649–57. doi:10.1158/1078-0432.CCR-10-0567. PMC 3513706. PMID 21372223.
- ^ Rane JK, Pellacani D, Maitland NJ (October 2012). "Advanced prostate cancer--a case for adjuvant differentiation therapy". Nature Reviews. Urology. 9 (10): 595–602. doi:10.1038/nrurol.2012.157. PMID 22890299. S2CID 43634798.
- ^ Jump up to: a b Jakob T, Tesfamariam YM, Macherey S, Kuhr K, Adams A, Monsef I, et al. (Cochrane Urology Group) (December 2020). "Bisphosphonates or RANK-ligand-inhibitors for men with prostate cancer and bone metastases: a network meta-analysis". The Cochrane Database of Systematic Reviews. 12: CD013020. doi:10.1002/14651858.CD013020.pub2. PMC 8095056. PMID 33270906.
- ^ Jump up to: a b "Surgery for Prostate Cancer". www.cancer.org. Retrieved 2020-03-30.
- ^ Jump up to: a b Ilic D, Evans SM, Allan CA, Jung JH, Murphy D, Frydenberg M (September 2017). "Laparoscopic and robotic-assisted versus open radical prostatectomy for the treatment of localised prostate cancer". The Cochrane Database of Systematic Reviews. 9: CD009625. doi:10.1002/14651858.cd009625.pub2. PMC 6486168. PMID 28895658.
- ^ "Continence management following prostate surgery". Continence Foundation of Australia. Archived from the original on 2020-04-10.
- ^ Singla N, Singla AK (March 2014). "Post-prostatectomy incontinence: Etiology, evaluation, and management". Turkish Journal of Urology. 40 (1): 1–8. doi:10.5152/tud.2014.222014. PMC 4548645. PMID 26328137.
- ^ "EAU Guidelines on Urinary Incontinence in Adults" (PDF). European Association of Urology. 2018.
- ^ "Erectile Dysfunction After Prostate Cancer". www.hopkinsmedicine.org. Retrieved 2020-04-01.
- ^ "Cancer of the Prostate - Cancer Stat Facts". seer.cancer.gov. Archived from the original on 18 March 2017. Retrieved 11 April 2017.
- ^ Wakai K (February 2005). "[Descriptive epidemiology of prostate cancer in Japan and Western countries]". Nihon Rinsho. Japanese Journal of Clinical Medicine (in Japanese). 63 (2): 207–12. PMID 15714967.
- ^ Jaubert de Beaujeu M, Chavrier Y (January 1976). "[Deformations of the anterior thoracic wall (author's transl)]". Annales de Chirurgie Thoracique et Cardio-Vasculaire (in French). 15 (1): 1–6. PMID 1259345.
- ^ Hsing AW, Tsao L, Devesa SS (January 2000). "International trends and patterns of prostate cancer incidence and mortality". International Journal of Cancer. 85 (1): 60–7. doi:10.1002/(SICI)1097-0215(20000101)85:1<60::AID-IJC11>3.0.CO;2-B. PMID 10585584.
- ^ Osegbe DN (April 1997). "Prostate cancer in Nigerians: facts and nonfacts". The Journal of Urology. 157 (4): 1340–3. doi:10.1016/S0022-5347(01)64966-8. PMID 9120935.
- ^ Bello JO (May 2017). "Predictors of survival outcomes in native sub Saharan black men newly diagnosed with metastatic prostate cancer". BMC Urology. 17 (1): 39. doi:10.1186/s12894-017-0228-0. PMC 5450414. PMID 28558685.
- ^ Di Blasio CJ, Rhee AC, Cho D, Scardino PT, Kattan MW (October 2003). "Predicting clinical end points: treatment nomograms in prostate cancer". Seminars in Oncology. 30 (5): 567–86. doi:10.1016/S0093-7754(03)00351-8. PMID 14571407.
- ^ Løvf M, Zhao S, Axcrona U, Johannessen B, Bakken AC, Carm KT, et al. (March 2019). "Multifocal Primary Prostate Cancer Exhibits High Degree of Genomic Heterogeneity". European Urology. 75 (3): 498–505. doi:10.1016/j.eururo.2018.08.009. PMID 30181068.
- ^ Hellerstedt BA, Pienta KJ (2002). "The current state of hormonal therapy for prostate cancer". Ca. 52 (3): 154–79. doi:10.3322/canjclin.52.3.154. PMID 12018929. S2CID 25311034.
- ^ Feldman BJ, Feldman D (October 2001). "The development of androgen-independent prostate cancer". Nature Reviews. Cancer. 1 (1): 34–45. doi:10.1038/35094009. PMID 11900250. S2CID 205020623.
- ^ Eifler JB, Feng Z, Lin BM, Partin MT, Humphreys EB, Han M, et al. (January 2013). "An updated prostate cancer staging nomogram (Partin tables) based on cases from 2006 to 2011". BJU International. 111 (1): 22–9. doi:10.1111/j.1464-410X.2012.11324.x. PMC 3876476. PMID 22834909.
- ^ "CaPSURE.net Home Page". 2006-09-27. Archived from the original on 2006-09-27. Retrieved 2020-08-08.
- ^ Cooperberg MR, Pasta DJ, Elkin EP, Litwin MS, Latini DM, Du Chane J, Carroll PR (June 2005). "The University of California, San Francisco Cancer of the Prostate Risk Assessment score: a straightforward and reliable preoperative predictor of disease recurrence after radical prostatectomy". The Journal of Urology. 173 (6): 1938–42. doi:10.1097/01.ju.0000158155.33890.e7. PMC 2948569. PMID 15879786.
- ^ Cooperberg MR, Freedland SJ, Pasta DJ, Elkin EP, Presti JC, Amling CL, et al. (November 2006). "Multiinstitutional validation of the UCSF cancer of the prostate risk assessment for prediction of recurrence after radical prostatectomy". Cancer. 107 (10): 2384–91. doi:10.1002/cncr.22262. PMID 17039503. S2CID 17420454.
- ^ May M, Knoll N, Siegsmund M, Fahlenkamp D, Vogler H, Hoschke B, Gralla O (November 2007). "Validity of the CAPRA score to predict biochemical recurrence-free survival after radical prostatectomy. Results from a european multicenter survey of 1,296 patients". The Journal of Urology. 178 (5): 1957–62, discussion 1962. doi:10.1016/j.juro.2007.07.043. PMID 17868719.
- ^ Zhao KH, Hernandez DJ, Han M, Humphreys EB, Mangold LA, Partin AW (August 2008). "External validation of University of California, San Francisco, Cancer of the Prostate Risk Assessment score". Urology. 72 (2): 396–400. doi:10.1016/j.urology.2007.11.165. PMID 18372031.
- ^ Cooperberg MR, Broering JM, Carroll PR (June 2009). "Risk assessment for prostate cancer metastasis and mortality at the time of diagnosis". Journal of the National Cancer Institute. 101 (12): 878–87. doi:10.1093/jnci/djp122. PMC 2697208. PMID 19509351.
- ^ "CDC FastStats". Centers for Disease Control. Archived from the original on 2017-07-28.
- ^ "Treatment Choices for Men With Early-Stage Prostate Cancer". National Cancer Institute. 2014-10-17. Archived from the original on 2015-04-04.
- ^ Eggener SE, Badani K, Barocas DA, Barrisford GW, Cheng JS, Chin AI, et al. (September 2015). "Gleason 6 Prostate Cancer: Translating Biology into Population Health". The Journal of Urology. 194 (3): 626–34. doi:10.1016/j.juro.2015.01.126. PMC 4551510. PMID 25849602.
- ^ "WHO Disease and injury country estimates". World Health Organization. 2009. Archived from the original on 2009-11-11. Retrieved Nov 11, 2009.
- ^ World Cancer Report 2014. International Agency for Research on Cancer, World Health Organization. ISBN 978-92-832-0432-9.
- ^ Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D (2011). "Global cancer statistics". Ca. 61 (2): 69–90. doi:10.3322/caac.20107. PMID 21296855. S2CID 30500384.
- ^ Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. (December 2012). "Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010". Lancet. 380 (9859): 2095–128. doi:10.1016/S0140-6736(12)61728-0. hdl:10536/DRO/DU:30050819. PMID 23245604. S2CID 1541253.
- ^ "Prostate Cancer Statistics". Laparoscopic Urology. Archived from the original on 24 June 2016. Retrieved 19 June 2016.
- ^ Jump up to: a b Overview: Prostate Cancer – What Causes Prostate Cancer? Archived 2006-04-04 at the Wayback Machine American Cancer Society (2 May 2006). Retrieved on 5 April 2007
- ^ Jump up to: a b Prostate Cancer FAQs. Archived 2006-05-29 at the Wayback Machine State University of New York School of Medicine Department of Urology (31 August 2006). Retrieved on 5 April 2007
- ^ Lee MM, Gomez SL, Chang JS, Wey M, Wang RT, Hsing AW (July 2003). "Soy and isoflavone consumption in relation to prostate cancer risk in China". Cancer Epidemiology, Biomarkers & Prevention. 12 (7): 665–8. PMID 12869409.
- ^ Potosky AL, Miller BA, Albertsen PC, Kramer BS (February 1995). "The role of increasing detection in the rising incidence of prostate cancer". JAMA. 273 (7): 548–52. doi:10.1001/jama.273.7.548. PMID 7530782.
- ^ Hanno P.M., Malcowicz S.B., Wein A.J., "Clinical Manual of Urology" McGraw Hill 2001
- ^ Homma Y, Kawabe K, Tsukamoto T, Yamanaka H, Okada K, Okajima E, et al. (January 1997). "Epidemiologic survey of lower urinary tract symptoms in Asia and Australia using the international prostate symptom score". International Journal of Urology. 4 (1): 40–6. doi:10.1111/j.1442-2042.1997.tb00138.x. PMID 9179665. S2CID 19636484.
- ^ Bostwick DG, Eble JN (2007). Urological Surgical Pathology. St. Louis: Mosby. p. 468. ISBN 978-0-323-01970-5.
- ^ Jump up to: a b Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, et al. (2005). "Cancer statistics, 2005". Ca. 55 (1): 10–30. doi:10.3322/canjclin.55.1.10. PMID 15661684. S2CID 22356919.
- ^ PDQ Screening and Prevention Editorial Board (2002). "Prostate Cancer Screening (PDQ®): Health Professional Version". PDQ Cancer Information Summaries. Bethesda (MD): National Cancer Institute (US). PMID 26389383.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, et al. (April 2013). "Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012". European Journal of Cancer. 49 (6): 1374–403. doi:10.1016/j.ejca.2012.12.027. PMID 23485231.
- ^ "Prostate cancer statistics". Cancer Research UK. Archived from the original on 6 October 2014. Retrieved 3 October 2014.
- ^ Ghabili K, Tosoian JJ, Schaeffer EM, Pavlovich CP, Golzari SE, Khajir G, et al. (November 2016). "The History of Prostate Cancer From Antiquity: Review of Paleopathological Studies". Urology. 97: 8–12. doi:10.1016/j.urology.2016.08.032. PMID 27591810.
- ^ Jump up to: a b Nahon I, Waddington G, Dorey G, Adams R (2011). "The history of urologic surgery: from reeds to robotics". Urologic Nursing. 31 (3): 173–80. doi:10.7257/1053-816X.2011.31.3.173. PMID 21805756.
- ^ Adams J (1853). "The case of scirrhous of the prostate gland with corresponding affliction of the lymphatic glands in the lumbar region and in the pelvis". Lancet. 1 (1547): 393–94. doi:10.1016/S0140-6736(02)68759-8.
- ^ Lytton B (June 2001). "Prostate cancer: a brief history and the discovery of hormonal ablation treatment". The Journal of Urology. 165 (6 Pt 1): 1859–62. doi:10.1016/S0022-5347(05)66228-3. PMID 11371867.
- ^ Samuel David Gross (1851). A Practical Treatise On the Diseases and Injuries of the Urinary Bladder, the Prostate Gland, and the Urethra. Philadelphia: Blanchard and Lea.
- ^ Young HH (1905). "Four cases of radical prostatectomy". Johns Hopkins Bull. 16.
- ^ Walsh PC, Lepor H, Eggleston JC (1983). "Radical prostatectomy with preservation of sexual function: anatomical and pathological considerations". The Prostate. 4 (5): 473–85. doi:10.1002/pros.2990040506. PMID 6889192. S2CID 30740301.
- ^ Huggins CB, Hodges CV (1941). "Studies on prostate cancer: 1. The effects of castration, of estrogen and androgen injection on serum phosphatases in metastatic carcinoma of the prostate". Cancer Res. 1 (4): 293. Archived from the original on 2017-06-30. Retrieved 2015-09-02.
- ^ Schally AV, Kastin AJ, Arimura A (November 1971). "Hypothalamic follicle-stimulating hormone (FSH) and luteinizing hormone (LH)-regulating hormone: structure, physiology, and clinical studies". Fertility and Sterility. 22 (11): 703–21. doi:10.1016/S0015-0282(16)38580-6. PMID 4941683.
- ^ Tolis G, Ackman D, Stellos A, Mehta A, Labrie F, Fazekas AT, et al. (March 1982). "Tumor growth inhibition in patients with prostatic carcinoma treated with luteinizing hormone-releasing hormone agonists". Proceedings of the National Academy of Sciences of the United States of America. 79 (5): 1658–62. Bibcode:1982PNAS...79.1658T. doi:10.1073/pnas.79.5.1658. PMC 346035. PMID 6461861.
- ^ Denmeade SR, Isaacs JT (May 2002). "A history of prostate cancer treatment". Nature Reviews. Cancer. 2 (5): 389–96. doi:10.1038/nrc801. PMC 4124639. PMID 12044015.
- ^ Scott WW, Johnson DE, Schmidt JE, Gibbons RP, Prout GR, Joiner JR, et al. (December 1975). "Chemotherapy of advanced prostatic carcinoma with cyclophosphamide or 5-fluorouracil: results of first national randomized study". The Journal of Urology. 114 (6): 909–11. doi:10.1016/S0022-5347(17)67172-6. PMID 1104900.
- ^ "FDA approves new drug for advanced prostate cancer" (Press release). Food and Drug Administration. 15 May 2013. Archived from the original on 4 June 2013.
- ^ Jump up to: a b Urisman A, Molinaro RJ, Fischer N, Plummer SJ, Casey G, Klein EA, et al. (March 2006). "Identification of a novel Gammaretrovirus in prostate tumors of patients homozygous for R462Q RNASEL variant". PLOS Pathogens. 2 (3): e25. doi:10.1371/journal.ppat.0020025. PMC 1434790. PMID 16609730. (Retracted, see doi:10.1371/annotation/7e2efc01-2e9b-4e9b-aef0-87ab0e4e4732)
- ^ Jump up to: a b c Arnst C (2007-06-13). "A Gender Gap in Cancer". Businessweek.com. Archived from the original on 2011-08-06. Retrieved 2011-08-29.
- ^ Browne A (2001-10-07). "Cancer bias puts breasts first". The Guardian. London. Archived from the original on 2016-12-26.
- ^ Templeton S (2005-10-16). "Men lose out in battle for cancer cash". Sunday Times. Archived from the original on 2011-05-24.
- ^ Farrell W, Sterba JP (2008). Does feminism discriminate against men? : a debate. ISBN 978-0-19-531283-6. Retrieved 2011-08-29.
- ^ National Prostate Cancer Coalition. The Prostate Cancer Gap. A Crisis in Men’s Health. National Prostate Cancer Coalition; 2007 Webcite
- ^ "Men, don't wait until there's a problem to see a doctor". Tampa Bay Times. 7 July 2016. Retrieved 2019-08-27.
- ^ Minium, Harry. "Men need to hear the story of ODU coach Jeff Jones' recovery from prostate cancer". The Virginian-Pilot. Retrieved 2019-08-27.
- ^ "Positive Outcome of Interim Analysis of pivotal Alpharadin study: Primary endpoint met in Phase III ALSYMPCA study" (Press release). Algeta ASA. 2011-06-06. Archived from the original on 2011-08-11. Retrieved 2011-07-04.CS1 maint: unfit URL (link)
- ^ Jump up to: a b Geethakumari PR, Cookson MS, Kelly WK (February 2016). "The Evolving Biology of Castration-Resistant Prostate Cancer: Review of Recommendations From the Prostate Cancer Clinical Trials Working Group 3". Oncology. 30 (2): 187–95, 199. PMID 26888794. Archived from the original on 22 February 2016.
- ^ Jump up to: a b Elshan NG, Rettig MB, Jung ME (May 2019). "Molecules targeting the androgen receptor (AR) signaling axis beyond the AR-Ligand binding domain". Medicinal Research Reviews. 39 (3): 910–960. doi:10.1002/med.21548. PMC 6608750. PMID 30565725.
- ^ Jump up to: a b c Ghosh J, Myers CE (October 1998). "Inhibition of arachidonate 5-lipoxygenase triggers massive apoptosis in human prostate cancer cells". Proceedings of the National Academy of Sciences of the United States of America. 95 (22): 13182–7. Bibcode:1998PNAS...9513182G. doi:10.1073/pnas.95.22.13182. PMC 23752. PMID 9789062.
Inhibition of 5-lipoxygenase by MK886 completely blocks 5-HETE production and induces massive apoptosis in both hormone-responsive (LNCaP) and -nonresponsive (PC3) human prostate cancer cells. This cell death is very rapid
- ^ Jump up to: a b c Greene ER, Huang S, Serhan CN, Panigrahy D (November 2011). "Regulation of inflammation in cancer by eicosanoids". Prostaglandins & Other Lipid Mediators. 96 (1–4): 27–36. doi:10.1016/j.prostaglandins.2011.08.004. PMC 4051344. PMID 21864702.
The 5-lipoxygenase (5-LOX) pathway is implicated in the development and progression of human cancers. 5-LOX, whose crystal structure was recently identified (118), is a key enzyme in metabolizing arachidonic acid to leukotrienes. 5-LOX can be induced by proinflammatory stimuli and is expressed in epithelial cancers including lung, prostate, breast, and colon (113). Hence, 5-LOX inhibitors have been targeted for their chemopreventive effects. Inhibition of 5-LOX activity is shown to block prostate cancer cell proliferation as well as carcinogen-induced lung tumorigenesis (119, 120). ... Both 5-HETE and 12-HETE are also products of lipoxygenase and are involved in tumor progression (12). Exogenous 5-HETE can stimulate the proliferation of prostate cancer cells and act as a survival factor (137, 138). These results require relatively high concentrations (at a concentration of 10 μM). Blocking the formation of 5-HETE, by inhibiting 5-lipoxygenase, results in massive apoptosis of human prostate cancer cells (139).
- ^ Jump up to: a b c Bishayee K, Khuda-Bukhsh AR (September 2013). "5-lipoxygenase antagonist therapy: a new approach towards targeted cancer chemotherapy". Acta Biochimica et Biophysica Sinica. 45 (9): 709–19. doi:10.1093/abbs/gmt064. PMID 23752617.
Recent studies demonstrated the involvement of growth factors, such as epidermal growth factor (EGF) and neurotensin in the 5-LOX-mediated tumor progression in prostate cancer [22,23]. Recent studies with 5-LOX siRNA [10] and specific blocker of 5-LOX [24] revealed the relation of this gene with the tumor cell proliferation. ... Meclofenamate sodium (MS) is known for its anti-inflammatory activity, and apart from this, Boctor et al. [37] reported that it caused reduction in the formation of 5-HETE in human leucocytes when used. MS can thus be considered a dual inhibitor of 5-LOX and COX pathways of arachidonic acid cascade. Further investigation with this substance revealed that it could interfere with the LT receptors in the lung carcinoma [38]. In a recent study, a group of scientists have shown the effect of MS on prostate cancer cells both in vitro and in vivo [39], and their result suggests a profound reduction in the tumor growth and cancer metastasis. ... While the commonly used inhibitors produced strong cytotoxicity, notably, zileuton, the only commercialized 5-LOX inhibitor, failed to induce an anti-proliferative or cytotoxic response in all other types of tumor cells where 5-LOX was in inactive state (e.g. HeLa cells). But where 5-LOX was in active state, zileuton could effectively inhibit progression, as in case of prostate cancer.
- ^ Martínez-Bosch N, Rodriguez-Vida A, Juanpere N, Lloreta J, Rovira A, Albanell J, et al. (July 2019). "Galectins in prostate and bladder cancer: tumorigenic roles and clinical opportunities". Nature Reviews. Urology. 16 (7): 433–445. doi:10.1038/s41585-019-0183-5. hdl:10261/201560. PMID 31015643. S2CID 128360958.
- ^ Munkley J, Mills IG, Elliott DJ (June 2016). "The role of glycans in the development and progression of prostate cancer". Nature Reviews. Urology. 13 (6): 324–33. doi:10.1038/nrurol.2016.65. PMID 27091662. S2CID 25916024.
- ^ Drake RR, Jones EE, Powers TW, Nyalwidhe JO (2015). "Altered glycosylation in prostate cancer". In Drake RR, Ball LE (eds.). Glycosylation and Cancer. Advances in Cancer Research Vol. 126. 126. pp. 345–82. doi:10.1016/bs.acr.2014.12.001. ISBN 9780128013816. PMID 25727153.
- ^ Laderach DJ, Gentilini LD, Giribaldi L, Delgado VC, Nugnes L, Croci DO, et al. (January 2013). "A unique galectin signature in human prostate cancer progression suggests galectin-1 as a key target for treatment of advanced disease". Cancer Research. 73 (1): 86–96. doi:10.1158/0008-5472.CAN-12-1260. PMID 23108139.
- ^ Compagno D, Gentilini LD, Jaworski FM, Pérez IG, Contrufo G, Laderach DJ (October 2014). "Glycans and galectins in prostate cancer biology, angiogenesis and metastasis". Glycobiology. 24 (10): 899–906. doi:10.1093/glycob/cwu055. PMID 24939371.
- ^ Linja MJ, Savinainen KJ, Saramäki OR, Tammela TL, Vessella RL, Visakorpi T (May 2001). "Amplification and overexpression of androgen receptor gene in hormone-refractory prostate cancer". Cancer Research. 61 (9): 3550–5. PMID 11325816.
- ^ Ford OH, Gregory CW, Kim D, Smitherman AB, Mohler JL (November 2003). "Androgen receptor gene amplification and protein expression in recurrent prostate cancer". The Journal of Urology. 170 (5): 1817–21. doi:10.1097/01.ju.0000091873.09677.f4. PMID 14532783.
- ^ Kokontis J, Takakura K, Hay N, Liao S (March 1994). "Increased androgen receptor activity and altered c-myc expression in prostate cancer cells after long-term androgen deprivation". Cancer Research. 54 (6): 1566–73. PMID 7511045.
- ^ Umekita Y, Hiipakka RA, Kokontis JM, Liao S (October 1996). "Human prostate tumor growth in athymic mice: inhibition by androgens and stimulation by finasteride". Proceedings of the National Academy of Sciences of the United States of America. 93 (21): 11802–7. Bibcode:1996PNAS...9311802U. doi:10.1073/pnas.93.21.11802. PMC 38139. PMID 8876218.
- ^ Kokontis JM, Hsu S, Chuu CP, Dang M, Fukuchi J, Hiipakka RA, Liao S (December 2005). "Role of androgen receptor in the progression of human prostate tumor cells to androgen independence and insensitivity". The Prostate. 65 (4): 287–98. doi:10.1002/pros.20285. PMID 16015608. S2CID 22349673.
- ^ Bourdoumis A, Papatsoris AG, Chrisofos M, Efstathiou E, Skolarikos A, Deliveliotis C (2010). "The novel prostate cancer antigen 3 (PCA3) biomarker". International Braz J Urol. 36 (6): 665–8, discussion 669. doi:10.1590/S1677-55382010000600003. PMID 21176272.
- ^ "Does PCA3 Testing for the Diagnosis and Management of Prostate Cancer improve patient health outcomes". www.cdc.gov. 2018-11-19. Retrieved 2020-08-09.
External links[]
| Classification | |
|---|---|
| External resources |
|
- Prostate cancer at Curlie
- Patient-centered information from the European Urological Association
- "Diagnosing and treating prostate cancer (video)". Mayo Clinic. February 2020.
- Prostate cancer
- Male genital neoplasia
- Neoplastic and hyperplastic prostate disorders
- Histopathology
- Infectious causes of cancer


