Goserelin
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Zoladex, others |
| Other names | D-Ser(But)6Azgly10-GnRH |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a601002 |
| Pregnancy category |
|
| Routes of administration | Implant |
| Drug class | GnRH analogue; GnRH agonist; Antigonadotropin |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | 27.3% |
| Elimination half-life | 4–5 hours |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.212.024 |
| Chemical and physical data | |
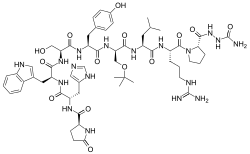
| Formula | C59H84N18O14 |
| Molar mass | 1269.433 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Goserelin, sold under the brand name Zoladex among others, is a medication which is used to suppress production of the sex hormones (testosterone and estrogen), particularly in the treatment of breast and prostate cancer.[1][2] It is an injectable gonadotropin releasing hormone agonist (GnRH agonist).
Structurally, it is a decapeptide. It is the natural GnRH decapeptide with two substitutions to inhibit rapid degradation.
Goserelin stimulates the production of the sex hormones testosterone and estrogen in a non-pulsatile (non-physiological) manner. This causes the disruption of the endogenous hormonal feedback systems, resulting in the down-regulation of testosterone and estrogen production.
It was patented in 1976 and approved for medical use in 1987.[3] It is on the World Health Organization's List of Essential Medicines.[4]
Medical uses[]

Goserelin is used to treat hormone-sensitive cancers of the breast (in pre- and peri-menopausal women) and prostate, and some benign gynaecological disorders (endometriosis, uterine fibroids and endometrial thinning). In addition, goserelin is used in assisted reproduction and in the treatment of precocious puberty. It may also be used in the treatment of male-to-female transgender people[5] and is favoured above other anti-androgens in some countries, such as the UK. It is available as a 1-month depot and a long-acting 3-month depot.
Goserelin is administered by subcutaneous injection as an implant every 28 days for the duration of treatment.[6]
Side effects[]
Goserelin may cause a temporary increase in bone pain and symptoms of prostatic cancer during the first few weeks of treatment. This is known as the tumour flare effect, and is the result of an initial increase in luteinizing hormone production, before the receptors are desensitised and hormonal production is inhibited. The symptoms will disappear, with hormonal inhibition. It is therefore advisable to co-treat with an antiandrogen during the first 2–3 weeks of goserelin treatment, particularly in patients with pre-existing bone symptoms.
Goserelin may cause bone pain, hot flushes, headache, stomach upset, depression, difficulty urinating (isolated cases), weight gain, swelling and tenderness of breasts (infrequent), decreased erections and reduced sexual desire. Bone pain can be managed symptomatically, and erectile dysfunction can be treated by vardenafil (Levitra) or other similar oral therapies, although they will not treat the reduced sexual desire. The rates of gynecomastia with goserelin have been found to range from 1 to 5%.[7]
Short-term memory impairment has also been reported in women and may in some cases be severe, but this effect disappears gradually once treatment is discontinued.[8][9]
Pharmacology[]
Goserelin is a synthetic analogue of a naturally occurring gonadotropin-releasing hormone (GnRH). Bioavailability is almost complete by injection. Goserelin is poorly protein-bound and has a serum elimination half-life of two to four hours in patients with normal renal function. The half-life increases with patients with impaired renal function. There is no significant change in pharmacokinetics in subjects with liver failure. After administration, peak serum concentrations are reached in about two hours. It rapidly binds to the GnRH receptor cells in the pituitary gland thus leading to an initial increase in production of luteinizing hormone and thus leading to an initial increase in the production of corresponding sex hormones. This initial flare may be treated by co-prescribing/co-administering an androgen receptor antagonist such as bicalutamide (Casodex). Eventually, after a period of about 14–21 days, production of LH is greatly reduced due to receptor downregulation, and sex hormones are generally reduced to castrate levels.[10]
Chemistry[]
Goserelin is a GnRH analogue and decapeptide. It is provided as the acetate salt.
Society and culture[]
Generic names[]
Goserelin is the generic name of the drug and its INN, USAN, and BAN.
References[]
- ^ Dictionary of Organic Compounds. CRC Press. 1996. pp. 3372–. ISBN 978-0-412-54090-5.
- ^ I.K. Morton; Judith M. Hall (6 December 2012). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. pp. 136–. ISBN 978-94-011-4439-1.
- ^ Fischer, Jnos; Ganellin, C. Robin (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 514. ISBN 9783527607495.
- ^ World Health Organization (2021). World Health Organization model list of essential medicines: 22nd list (2021). Geneva: World Health Organization. hdl:10665/345533. WHO/MHP/HPS/EML/2021.02.
- ^ Dittrich R, Binder H, Cupisti S, Hoffmann I, Beckmann MW, Mueller A. Endocrine treatment of male-to-female transsexuals using gonadotropin-releasing hormone agonist. Exp Clin Endocrinol Diabetes 2005;113:586–92.
- ^ "Goserelin". NICE (National Institute for Health and Care Excellence). 2016. Retrieved 19 November 2016.
- ^ Di Lorenzo G, Autorino R, Perdonà S, De Placido S (December 2005). "Management of gynaecomastia in patients with prostate cancer: a systematic review". Lancet Oncol. 6 (12): 972–9. doi:10.1016/S1470-2045(05)70464-2. PMID 16321765.
- ^ Newton CR, Yuzpe AA, Timmon IS, Slota MD. Memory complaints: a side effect of continued exposure to gonadotropin-releasing hormone agonists (GnRHa). Paper presented at: Conjoint Annual Meetings of the American Fertility Society and the Canadian Fertility and Andrology Society; October 11–14, 1993; Montreal, Canada.
- ^ Friedman AJ, Juneau-Norcross M, Rein MS. Adverse effects of leuprolide acetate depot treatment. Fertil Steril. 1993;59(2):448-450.
- ^ Kotake, Toshihiko; Michiyuki Usami; et al. (August 1999). "Goserelin Acetate with or without Antiandrogen or Estrogen in the Treatment of Patients with Advanced Prostate Cancer: a Multicenter, Randomized, Controlled Trial in Japan". Japanese Journal of Clin. Oncol. 29 (11): 562–570. doi:10.1093/jjco/29.11.562. ISSN 1465-3621. PMID 10678560.
External links[]
- AstraZeneca brands
- GnRH agonists
- Peptides
- Feminizing hormone therapy
- World Health Organization essential medicines