Linzagolix
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Yselty |
| Other names | KLH-2109; OBE-2109 |
| Routes of administration | By mouth[1] |
| Drug class | GnRH modulator; GnRH antagonist; Antigonadotropin |
| ATC code | |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL |
|
| Chemical and physical data | |
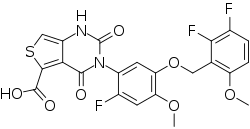
| Formula | C22H15F3N2O7S |
| Molar mass | 508.42 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Linzagolix (trade name Yselty) is a small-molecule, non-peptide, orally active gonadotropin-releasing hormone antagonist (GnRH antagonist) which is under development by Kissei Pharmaceutical and ObsEva for the treatment of uterine fibroids, endometriosis, and adenomyosis.[2][3][1] As of December 2020, it is under review for approval for uterine fibroids, is in phase III clinical trials for endometriosis, and is in phase II clinical studies for adenomyosis.[2]
Society and culture[]
Legal status[]
On 16 December 2021, the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) adopted a positive opinion, recommending the granting of a marketing authorization for the medicinal product Yselty, intended for the treatment of symptoms of uterine fibroids.[4] The applicant for this medicinal product is ObsEva Ireland Ltd.[4]
References[]
- ^ a b Ezzati M, Carr BR (2015). "Elagolix, a novel, orally bioavailable GnRH antagonist under investigation for the treatment of endometriosis-related pain". Womens Health (Lond). 11 (1): 19–28. doi:10.2217/whe.14.68. PMID 25581052.
- ^ a b "Linzagolix - Kissei Pharmaceutical/ObsEva - AdisInsight".
- ^ Chodankar, Rohan; Allison, Jennifer (2018). "New Horizons in Fibroid Management". Current Obstetrics and Gynecology Reports. 7 (2): 106–115. doi:10.1007/s13669-018-0242-6. ISSN 2161-3303.
- ^ a b "Yselty: Pending EC decision". European Medicines Agency. 16 December 2021. Retrieved 18 December 2021. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
Further reading[]
- Dababou S, Garzon S, Laganà AS, Ferrero S, Evangelisti G, Noventa M, et al. (September 2021). "Linzagolix: a new GnRH-antagonist under investigation for the treatment of endometriosis and uterine myomas". Expert Opin Investig Drugs. 30 (9): 903–11. doi:10.1080/13543784.2021.1957830. PMID 34278887. S2CID 236091261.
External links[]
- "Linzagolix". Drug Information Portal. U.S. National Library of Medicine.
- Carboxylic acids
- Ethers
- Experimental drugs
- Fluoroarenes
- GnRH antagonists
- Pyrimidines
- Triketones
- Systemic hormonal preparation stubs