Triptorelin
 | |
| Clinical data | |
|---|---|
| Trade names | Decapeptyl, Gonapeptyl, Triptodur, others |
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| Pregnancy category |
|
| Drug class | GnRH analogue; GnRH agonist; Antigonadotropin |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Excretion | Kidney |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.165.044 |
| Chemical and physical data | |
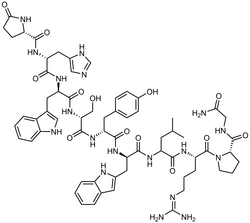
| Formula | C64H82N18O13 |
| Molar mass | 1311.473 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Triptorelin, sold under the brand names Decapeptyl and Gonapeptyl among others, is a medication that acts as an agonist analog of gonadotropin-releasing hormone, thus reversibly repressing expression of luteinizing hormone (LH) and follicle-stimulating hormone (FSH).[1][2]
It is a decapeptide (pGlu-His-Trp-Ser-Tyr-D-Trp-Leu-Arg-Pro-Gly-NH2) and a gonadotropin-releasing hormone agonist (GnRH agonist) used as the acetate or pamoate salts.
Primary indications include endometriosis, for the reduction of fibroids, for prostatic cancer, and to treat male hypersexuality with severe sexual deviation.[2] The drug has also been used off label to delay puberty in patients with gender dysphoria.[3]
It was patented in 1975 and approved for medical use in 1986.[4] It is on the World Health Organization's List of Essential Medicines.[5]
Medical uses[]
Triptorelin is used to treat prostate cancer as part of androgen deprivation therapy.[6]
Another common use in the United Kingdom is for hormone replacement therapy to suppress testosterone or estrogen levels in transgender people (in conjunction with estradiol valerate for trans women or testosterone for trans men). Spironolactone and cyproterone acetate are other drugs used by trans people to suppress sex hormones, but these drugs have a completely different mechanism of action.[7] It can also be used as a puberty blocker.[8]
Triptorelin has been used as a chemical castration agent for reducing sexual urges in sex offenders.[9]
Drug action[]
Triptorelin is a gonadorelin analogue, also known as luteinizing hormone releasing analogue (GnRH analogue, LHRH analogue).[1] The drug binds to receptors in the pituitary gland and stimulates secretion of gonadotropins (namely luteinzing hormone and follicle-stimulating hormone). This causes an initial phase of LH and FSH stimulation, prior to down-regulation of the gonadotrophin-releasing hormone receptors, thereby reducing the release of gonadotropins in the long term, which in turn leads to the inhibition of androgen and oestrogen production.[2]
Side-effects[]
General side effects can include:[2]
- Anaphylaxis
- Arthralgia
- Asthenia
- Asthma
- Breast tenderness (males and females)
- Changes in Blood pressure
- Changes in breast size
- Depression
- Ovarian cysts
- Mood changes
- Skin rashes
- Hot flushes
- Weight changes
Contra-indications and precautions[]
Triptorelin should not be used for more than 6 months for endometriosis,[2] and should be used with care in patients with metabolic bone disease and osteoporosis due to the risk of osteopenia.[2]
Society and culture[]
Brand names[]
Triptorelin is marketed under the brand names Decapeptyl (Ipsen) and Diphereline and Gonapeptyl (Ferring Pharmaceuticals). In the United States, it is sold by Watson Pharmaceuticals as Trelstar and by Arbor Pharmaceuticals as Triptodur (an extended-release 6-month depot injection). In Iran, triptorelin is marketed under the brand name Variopeptyl.
Research[]
Triptorelin and other antiandrogens may be effective in the treatment of obsessive–compulsive disorder.[10]
See also[]
- Gonadotropin-releasing hormone receptor § Agonists
References[]
- ^ a b "gonadorelin analogue | Encyclopedia.com". www.encyclopedia.com. Retrieved 2019-09-21.
- ^ a b c d e f Joint Formulary Committee (2018). British National Formulary (BNF) 70. London: Pharmaceutical Press. p. 635. ISBN 978-0-85711-173-9.
- ^ Barnes, Hannah; Cohen, Deborah (2019-09-20). "Gender dysphoria in children: puberty blockers study draws further criticism". BMJ. 366: l5647. doi:10.1136/bmj.l5647. ISSN 0959-8138. PMID 31540909. S2CID 202711942.
- ^ Fischer, Jnos; Ganellin, C. Robin (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 514. ISBN 9783527607495.
- ^ World Health Organization (2021). World Health Organization model list of essential medicines: 22nd list (2021). Geneva: World Health Organization. hdl:10665/345533. WHO/MHP/HPS/EML/2021.02.
- ^ "triptorelin (Intramuscular route)". drugs.com. Retrieved 11 November 2016.
- ^ Wylie, Kevan Richard; Fung, Robert; Boshier, Claudia; Rotchell, Margaret (2009). "Recommendations of endocrine treatment for patients with gender dysphoria". Sexual and Relationship Therapy. 24 (2): 175–187. doi:10.1080/14681990903023306. S2CID 20471537.
- ^ Shumer, DE; Nokoff, NJ; Spack, NP (August 2016). "Advances in the Care of Transgender Children and Adolescents". Advances in Pediatrics. 63 (1): 79–102. doi:10.1016/j.yapd.2016.04.018. PMC 4955762. PMID 27426896.
- ^ Study: Drug effectively treats pedophilia, CNN, February 11, 1998.
- ^ Nomani H, Mohammadpour AH, Moallem SM, YazdanAbad MJ, Barreto GE, Sahebkar A (December 2019). "Anti-androgen drugs in the treatment of obsessive-compulsive disorder: a systematic review". Curr Med Chem. 27 (40): 6825–6836. doi:10.2174/0929867326666191209142209. PMID 31814547. S2CID 208956450.
Further reading[]
- Lahlou N, Carel JC, Chaussain JL, Roger M (July 2000). "Pharmacokinetics and pharmacodynamics of GnRH agonists: clinical implications in pediatrics". J Pediatr Endocrinol Metab. 13 Suppl 1: 723–37. doi:10.1515/jpem.2000.13.s1.723. PMID 10969915. S2CID 3639804.
- Padula AM (August 2005). "GnRH analogues—agonists and antagonists". Anim Reprod Sci. 88 (1–2): 115–26. doi:10.1016/j.anireprosci.2005.05.005. PMID 15955640.
- GnRH agonists
- Peptides
- Puberty blockers
- AbbVie brands
- World Health Organization essential medicines