Pacemaker potential
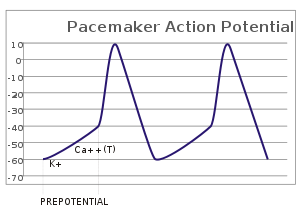
In the pacemaking cells of the heart (e.g., the sinoatrial node), the pacemaker potential (also called the pacemaker current) is the slow, positive increase in voltage across the cell's membrane (the membrane potential) that occurs between the end of one action potential and the beginning of the next action potential. This increase in membrane potential is what causes the cell membrane, which typically maintains a resting membrane potential of -70 mV,[1] to reach the threshold potential and consequently fire the next action potential; thus, the pacemaker potential is what drives the self-generated rhythmic firing (automaticity) of pacemaker cells, and the rate of change (i.e., the slope) of the pacemaker potential is what determines the timing of the next action potential and thus the intrinsic firing rate of the cell. In a healthy sinoatrial node (SAN, a complex tissue within the right atrium containing pacemaker cells that normally determine the intrinsic firing rate for the entire heart[2][3]), the pacemaker potential is the main determinant of the heart rate. Because the pacemaker potential represents the non-contracting time between heart beats (diastole), it is also called the diastolic depolarization. The amount of net inward current required to move the cell membrane potential during the pacemaker phase is extremely small, in the order of few pAs, but this net flux arises from time to time changing contribution of several currents that flow with different voltage and time dependence. Evidence in support of the active presence of K+, Ca2+, Na+ channels and Na+/K+ exchanger during the pacemaker phase have been variously reported in the literature, but several indications point to the “funny”(If) current as one of the most important.[4](see funny current). There is now substantial evidence that also sarcoplasmic reticulum (SR) Ca2+-transients participate to the generation of the diastolic depolarization via a process involving the Na–Ca exchanger.
The rhythmic activity of some neurons like the pre-Bötzinger complex is modulated by neurotransmitters and neuropeptides, and such modulatory connectivity gives to the neurons the necessary plasticity to generating distinctive, state-dependent rhythmic patterns that depend on pacemarker potentials.[5]
Distinctions among autonomic foci[]
In reality, the heart has several pacemakers known as autonomic foci, each which fires at its own intrinsic rate:
- SA node: 60–100 bpm
- Atrial foci: 60–80 bpm
- Junctional foci: 40–60 bpm
- Ventricular foci: 20–40 bpm
The potentials will normally travel in order
SA node → atrial foci → junctional foci → ventricular foci
Pacemaker potentials are fired not only by SA node, but also by the other foci. However, the other firing frequencies are slower than the one of the SA node (as seen above). Normally, all the foci will end up firing at the SA node rate, not their intrinsic rate. The other foci attempt to fire at their intrinsic rate, but they are activated by the SA node before they can fire. This rapid firing causes all the foci to fire faster than their intrinsic rates, a phenomenon known as overdrive-suppression. Thus, in the normal, healthy heart, only the SA node intrinsic rate is observable.
Pathology[]
However, in pathological conditions, the intrinsic rate becomes apparent. Consider a heart attack which damages the region of the heart between the SA node and the atrial foci.
SA node → |block| atrial foci → junctional foci → ventricular foci
The other foci will not see the SA node firing; however, they will see the atrial foci. The heart will now beat at the intrinsic rate of the atrial foci.
Induction[]
The firing of the pacemaker cells is induced electrically by reaching the threshold potential of the cell membrane. The threshold potential is the potential an excitable cell membrane, such as a myocyte, must reach in order to induce an action potential.[6] This depolarization is caused by very small net inward currents of calcium ions across the cell membrane, which gives rise to the action potential.[7][8]
Bio-pacemakers[]
Bio-pacemakers are the outcome of a rapidly emerging field of research into a replacement for the electronic pacemaker. The bio-pacemaker turns quiescent myocardial cells (e.g. atrial cells) into pacemaker cells. This is achieved by making the cells express a gene which creates a pacemaker current.[9]
See also[]
References[]
- ^ Berne, Robert; Matthew Levy; Bruce Koeppen; Bruce Stanton (2004). Physiology. Elsevier Mosby. p. 276. ISBN 978-0-8243-0348-8.
- ^ Verkerk AO, van Boren MM, Peters RJ, Broekhuis E, Lam K, Coronel R, de Bakker JM, Tan HR (October 2007). "Pacemaker current (I)f)) in the human sinoatrial node". Eur Heart J. 28 (1): 2472–8. doi:10.1093/eurheartj/ehm339. PMID 17823213.
- ^ Boron, Walter. F; Emile Boulpaep (2003). Medical Physiology. Elsevier Saunders. p. 489. ISBN 978-0-7216-0076-5.
- ^ DiFrancesco D (May 2006). "Funny channels in the control of cardiac rhythm and mode of action of selective blockers". Pharmacol. Res. 53 (5): 399–406. doi:10.1016/j.phrs.2006.03.006. PMID 16638640.
- ^ Morgado-Valle, Consuelo; Beltran-Parrazal, Luis (2017). "Respiratory Rhythm Generation: The Whole Is Greater Than the Sum of the Parts". Advances in Experimental Medicine and Biology. 1015: 147–161. doi:10.1007/978-3-319-62817-2_9. ISBN 978-3-319-62815-8. ISSN 0065-2598. PMID 29080026.
- ^ Campbell, Neil. A (1996). Biology. Benjamin Cummings. p. G–21. ISBN 978-0-07-366175-9.
- ^ Verkerk AO, van Ginneken AC, Wilders R (January 2009). "Pacemaker activity of the human sinoatrial node: Role of the hyperpolarization-activated current, I(f)". Int J Cardiol. 132 (3): 318–36. doi:10.1016/j.ijcard.2008.12.196. PMID 19181406.
- ^ Boron, Walter. F; Emile Boulpaep (2003). Medical Physiology. Elsevier Saunders. p. 487. ISBN 978-0-7216-0076-5.
- ^ Verkerk AO, Zegers JG, Van Ginneken AC, Wilders R (2008). "Dynamic action potential clamp as a powerful tool in the development of a gene-based bio-pacemaker". Conf Proc IEEE Eng Med Biol Soc. 1: 133–6. doi:10.1109/IEMBS.2008.4649108. ISBN 978-1-4244-1814-5. PMID 19162611.
- Cardiac electrophysiology
- Graded potentials