Photocatalysis

In chemistry, photocatalysis is the acceleration of a photoreaction in the presence of a catalyst.[1] In catalysed photolysis, light is absorbed by an adsorbed substrate. In photogenerated catalysis, the photocatalytic activity (PCA) depends on the ability of the catalyst to create electron–hole pairs, which generate free radicals (e.g. hydroxyl radicals: •OH) able to undergo secondary reactions. Its practical application was made possible by the discovery of water electrolysis by means of titanium dioxide (TiO2).
History[]
Early mentions of photocatalysis (1911–1938)[]
The earliest mention of photocatalysis dates back to 1911, when German chemist Dr. Alexander Eibner integrated the concept in his research of the illumination of zinc oxide (ZnO) on the bleaching of the dark blue pigment, Prussian blue.[2][3] Around this time, Bruner and Kozak published an article discussing the deterioration of oxalic acid in the presence of uranyl salts under illumination,[3][4] while in 1913, Landau published an article explaining the phenomenon of photocatalysis. Their contributions led to the development of actinometric measurements, measurements that provide the basis of determining photon flux in photochemical reactions.[3][5] After a brief stint of lack of research on photocatalysis, in 1921, Baly et al. used ferric hydroxides and colloidal uranium salts as catalysts for the creation of formaldehyde under light in the visible spectrum.[3][6] However, it wasn't until 1938, when Doodeve and Kitchener discovered that TiO2, a highly-stable and non-toxic oxide, in the presence of oxygen, could act as a photosensitizer for bleaching dyes, as ultraviolet light absorbed by TiO2 led to the production of active oxygen species on its surface, resulting in the blotching of organic chemicals via photooxidation. This would actually mark the first observation of the fundamental characteristics of heterogeneous photocatalysis.[3][7]
Advancements in photocatalysis research (1964–1981, present)[]
Research in photocatalysis subsided for over 25 years due to lack of interest and absence of practical applications. However, in 1964, V.N. Filimonov investigated isopropanol photooxidation from ZnO and TiO2;[3][8] at around the same time, Kato and Mashio, Doerffler and Hauffe, and Ikekawa et al. (1965) explored oxidation/photooxidation of carbon dioxide and organic solvents from ZnO radiance.[3][9][10][11] A few years later, in 1970, Formenti et al. and Tanaka and Blyholde observed the oxidation of various alkenes and the photocatalytic decay of nitrous oxide (N2O), respectively.[3][12][13]
However, a breakthrough in photocatalysis research occurred in 1972, when Akira Fujishima and Kenichi Honda discovered electrochemical photolysis of water occurring between connected TiO2 and platinum electrodes, in which ultraviolet light was absorbed by the former electrode, and electrons would flow from the TiO2 electrode (anode; site of oxidation reaction) to the platinum electrode (cathode; site of reduction reaction); with hydrogen production occurring at the cathode. This was one of the first instances in which hydrogen production could come from a clean and cost-effective source, as the majority of hydrogen production back then came – and still today comes – from natural gas reforming and gasification.[3][14] Fujishima's and Honda's findings have led to other advancements in photocatalysis; in 1977, Nozik discovered that the incorporation of a noble metal in the electrochemical photolysis process, such as platinum and gold, among others, could increase photoactivity, and that an external potential wasn't required.[3][15] Future research conducted by Wagner and Somorjai (1980) and Sakata and Kawai (1981) delineated the production of hydrogen on the surface of strontium titanate (SrTiO3) via photogeneration, and the generation of hydrogen and methane from the illumination of TiO2 and PtO2 in ethanol, respectively.[3][16][17]
Research and development in photocatalysis, especially in electrochemical photolysis of water, continues today, but so far, nothing has been developed for commercial purposes. In 2017, Chu et al. assessed the future of electrochemical photolysis of water, discussing its major challenge of developing a cost-effective, energy-efficient photoelectrochemical (PEC) tandem cell, which would, “mimic natural photosynthesis.”[3][18]
Types of photocatalysis[]
Homogeneous photocatalysis[]
In homogeneous photocatalysis, the reactants and the photocatalysts exist in the same phase. The most commonly used homogeneous photocatalysts include ozone and photo-Fenton systems (Fe+ and Fe+/H2O2). The reactive species is the •OH which is used for different purposes. The mechanism of hydroxyl radical production by ozone can follow two paths.[19]
- O3 + hν → O2 + O(1D)
- O(1D) + H2O → •OH + •OH
- O(1D) + H2O → H2O2
- H2O2 + hν → •OH + •OH
Similarly, the Fenton system produces hydroxyl radicals by the following mechanism[20]
- Fe2+ + H2O2→ HO• + Fe3+ + OH−
- Fe3+ + H2O2→ Fe2+ + HO•2 + H+
- Fe2+ + HO• → Fe3+ + OH−
In photo-Fenton type processes, additional sources of OH radicals should be considered: through photolysis of H2O2, and through reduction of Fe3+ ions under UV light:
- H2O2 + hν → HO• + HO•
- Fe3+ + H2O + hν → Fe2+ + HO• + H+
The efficiency of Fenton type processes is influenced by several operating parameters like concentration of hydrogen peroxide, pH and intensity of UV. The main advantage of this process is the ability of using sunlight with light sensitivity up to 450 nm, thus avoiding the high costs of UV lamps and electrical energy. These reactions have been proven more efficient than the other photocatalysis but the disadvantages of the process are the low pH values which are required, since iron precipitates at higher pH values and the fact that iron has to be removed after treatment.
Homogeneous photocatalysis can also be conducted by the Cu(II)/Cu(I) complexes.The photoredox behaviour of the Cu(II) complexes, similar to the Fe(III) ones, is derived mostly from the reactive decay of their LMCT states. Excitation to LMCT excited states can be attainable by direct sunlight, when the ionization energy of the ligands coordinated to Cu(II) is not very high. In consequence of the reactive decay of the LMCT excited state by inner-sphere electron transfer, the Cu(II) central atom is reduced to Cu(I), whereas ligand is oxidized to its radical and leaves the coordination sphere.[21]
The photoredox behaviour is demonstrated by the simple Cu(II) complexes with halogens. After excitation of [CuClx] 2−x the metal centre is reduced and Cl• and Cl2 •− radicals are formed.[22]
The Cl2 •− radicals are strong oxidation and chlorination agents. For instance they are able to oxidize phenol and its derivatives to para- and CO2.
Heterogeneous photocatalysis[]
Heterogeneous catalysis has the catalyst in a different phase from the reactants. Heterogeneous photocatalysis is a discipline which includes a large variety of reactions: mild or total oxidations, dehydrogenation, hydrogen transfer, 18O2–16O2 and deuterium-alkane isotopic exchange, metal deposition, water detoxification, gaseous pollutant removal, etc.
Most common heterogeneous photocatalysts are transition metal oxides and semiconductors, which have unique characteristics. Unlike the metals which have a continuum of electronic states, semiconductors possess a void energy region where no energy levels are available to promote recombination of an electron and hole produced by photoactivation in the solid. The void region, which extends from the top of the filled valence band to the bottom of the vacant conduction band, is called the band gap.[24] When a photon with energy equal to or greater than the materials band gap is absorbed by the semiconductor, an electron is excited from the valence band to the conduction band, generating a positive hole in the valence band. Such a photogenerated electron-hole pair is termed an exciton. The excited electron and hole can recombine and release the energy gained from the excitation of the electron as heat. Exciton recombination is undesirable and higher levels lead to an inefficient photocatalyst. For this reason efforts to develop functional photocatalysts often emphasize extending exciton lifetime, improving electron-hole separation using diverse approaches that often rely on structural features such as phase hetero-junctions (e.g. anatase-rutile interfaces), noble-metal nanoparticles, silicon nanowires and substitutional cation doping.[25] The ultimate goal of photocatalyst design is to facilitate reactions between the excited electrons with oxidants to produce reduced products, and/or reactions between the generated holes with reductants to produce oxidized products. Due to the generation of positive holes and electrons, oxidation-reduction reactions take place at the surface of semiconductors.
In one mechanism of the oxidative reaction, the positive holes react with the moisture present on the surface and produce a hydroxyl radical. The reaction starts by photo-induced exciton generation in the metal oxide surface (MO stands for metal oxide):
- MO + hν → MO (h+ + e−)
Oxidative reactions due to photocatalytic effect:
- h+ + H2O → H+ + •OH
- 2 h+ + 2 H2O → 2 H+ + H2O2
- H2O2→ 2 •OH
Reductive reactions due to photocatalytic effect:
- e− + O2 → •O2−
- •O2− + H2O + H+ → H2O2 + O2
- H2O2 → 2 •OH
Ultimately, the hydroxyl radicals are generated in both the reactions. These hydroxyl radicals are very oxidative in nature and non selective with redox potential of (E0 = +3.06 V)[26]
TiO2 is a good and common choice for heterogeneous catalysis. Inertness to chemical environment and long-term photostability has made TiO2 an important material in many practical applications. Large bandgap semiconductors like TiO2 are commonly investigated in the rutile (bandgap 3.0 eV) and anatase (bandgap 3.2 eV) phases. Photocatalytic reactions are initiated by the absorption of illumination with energy equal to or greater than the band gap of the semiconductor. This produces electron-hole (e− /h+ ) pairs:[27]
Where the electron is in the conduction band and the hole is in the valence band. TiO2 particle can behave either as an electron donor or acceptor for molecules in contact with the semiconductor. They can participate in redox reactions with adsorbed species as the valence band hole is strongly oxidizing while the conduction band electron is strongly reducing.[27]
Applications[]
- Paper production: Large scale photocatalysis by micro-sized ZnO tetrapodal particles added to pilot paper production.[28] The most common are one-dimensional nanostructures, such as nanorods, nanotubes, nanofibers, nanowires, but also nanoplates, nanosheets, nanospheres, tetrapods. ZnO has a strong oxidation ability, chemical stability, enhanced photocatalytic activity, and a large free-exciton binding energy. Also, it is non-toxic, earth abundant, biocompatible, biodegradable, environmentally friendly, low cost, and compatible with simple chemical synthesis. ZnO has also restrictions to its widespread use in photocatalysis under solar radiation as previously mentioned. Thus, several approaches have been suggested to overcome this limitation, including nonmetal and metal doping for reducing the band gap and improving the charge carrier separation.[29]
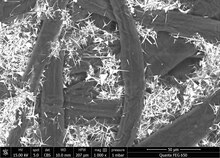 SEM image of wood pulp (dark fibers) and tetrapodal zinc oxide micro particles (white and spiky) i a paper.[28]
SEM image of wood pulp (dark fibers) and tetrapodal zinc oxide micro particles (white and spiky) i a paper.[28] - Separating water into hydrogen and oxygen by photocatalytic water splitting.[30] Usage of fossil fuels is causing a huge amount of air pollutants, such as nitrogen oxides, sulfur oxides,[31][1] and carbon oxides.[32] Using sunlight as a renewable energy source is therefore becoming increasingly interesting.[33] In order to continue exploring photocatalytic hydrogen production efficiency, the most prevalently investigated titanium dioxide (TiO2) which has limited photocatalytic hydrogen production efficiency, was further loaded with different amounts of nickel oxide (NiO). From the results obtained, it can be cоncluded that the addition of NiO leads tо a significant explоitation of the visible part оf the spectrum.[34] An efficient photocatalyst in the UV range is based on sodium tantalite (NaTaO3) doped with lanthanum and loaded with a cocatalyst nickel oxide. The surface of the sodium tantalite crystals is grooved with nanosteps that are a result of doping with lanthanum (3–15 nm range, see nanotechnology). The NiO particles which facilitate hydrogen gas evolution are present on the edges, with the oxygen gas evolving from the grooves.
- Use of titanium dioxide in self-cleaning glass. Free radicals[35][36] generated from TiO2 oxidize organic matter.[37][38] The rough wedge-like TiO2 surface was subsequently modified with a hydrophobic monolayer of octadecylphosphonic acid (ODP). TiO2 surfaces plasma etched for 10 second and subsequent surface modification with ODP showed a WCA greater than 150◦. The surface was converted into a superhydrophilic surface (Water Contact Angle = 0◦) upon UV illumination, due to rapid decomposition of octadecylphosphonic acid coating resulting from TiO2 photocatalysis. Due to the wide band gap of TiO2, light absorption by the semiconductor material and consequently superhydrophilic conversion of undoped TiO2 are limited to only ultraviolet region (wavelength <390 nm) and thereby restricting the practical uses of self-cleaning phenomenon to outdoor applications only.[39]
- Disinfection of water by supported titanium dioxide photocatalysts,[40] a form of solar water disinfection (SODIS).[41][42]
- Use of titanium dioxide in self-sterilizing photocatalytic coatings (for application to food contact surfaces and in other environments where microbial pathogens spread by indirect contact).[43]
- Oxidation of organic contaminants using magnetic particles that are coated with titanium dioxide nanoparticles and agitated using a magnetic field while being exposed to UV light.[44]
- Conversion of carbon dioxide into gaseous hydrocarbons using titanium dioxide in the presence of water.[45] As an efficient absorber in the UV range, titanium dioxide nanoparticles in the anatase and rutile phases are able to generate excitons by promoting electrons across the band gap. The electrons and holes react with the surrounding water vapor to produce hydroxyl radicals and protons. At present, proposed reaction mechanisms usually suggest the creation of a highly reactive carbon radical from carbon monoxide and carbon dioxide which then reacts with the photogenerated protons to ultimately form methane. Although the efficiencies of present titanium dioxide based photocatalysts are low, the incorporation of carbon based nanostructures such as carbon nanotubes[46] and metallic nanoparticles[47] have been shown to enhance the efficiency of these photocatalysts.
- Sterilization of surgical instruments and removal of unwanted fingerprints from sensitive electrical and optical components.[48]
- A less-toxic alternative to tin and copper-based antifouling marine paints, , generates hydrogen peroxide by photocatalysis.
- Antifouling coatings for filtration membranes, which can also act as separation layer[49] and photocatalyst for degradation of contaminants of emerging concern.[50] or Cr(VI) removal.[51]
- Decomposition of crude oil with TiO2 nanoparticles: by using titanium dioxide photocatalysts and UV-A radiation from the sun, the hydrocarbons found in crude oil can be turned into H2O and CO2. Higher amounts of oxygen and UV radiation increased the degradation of the model organics. These particles can be placed on floating substrates, making it easier to recover and catalyze the reaction. This is relevant since oil slicks float on top of the ocean and photons from the sun target the surface more than the inner depth of the ocean. By covering floating substrates like woodchips with epoxy adhesives, water logging can be prevented and TiO2 particles can stick to the substrates. With more research, this method should be applicable to other organics.
- Decontamination of water with photocatalysis and adsorption: the removal and destruction of organic contaminants in groundwater can be addressed through the impregnation of adsorbents with photoactive catalysts. These adsorbents attract contaminating organic atoms/molecules like tetrachloroethylene to them. The photoactive catalysts impregnated inside speed up the degradation of the organics. Adsorbents are placed in packed beds for 18 hours, which would attract and degrade the organic compounds. The spent adsorbents would then be placed in regeneration fluid, essentially taking away all organics still attached by passing hot water counter-current to the flow of water during the adsorption process to speed up the reaction. The regeneration fluid then gets passed through the fixed beds of silica gel photocatalysts to remove and decompose the rest of the organics left. Through the use of fixed bed reactors, the regeneration of adsorbents can help increase the efficiency.
- Some photoactive catalysts have been introduced over the last decade, such as TiO2 and ZnO nano rodes. Most of them suffer from the fact that they can only perform under UV irradiation due to their band structure hence some other photocatalysis have been introduced over the last few years to encounter this problem like Graphene-ZnO nanocompound.[52]
- Decomposition of polyaromatic hydrocarbons (PAHs). Triethylamine (TEA) was utilized to solvate and extract the polyaromatic hydrocarbons (PAHs) found in crude oil. By solvating these PAHs, TEA can attract the PAHs to itself. Once removed, TiO2 slurries and UV light can photocatalytically degrade the PAHs. The figure shows the high success rate of this experiment. With high yielding of recoveries of 93–99% of these contaminants, this process has become an innovative idea that can be finalized for actual environmental usage. This procedure demonstrates the ability to develop photocatalysts that would be performed at ambient pressure, ambient temperature, and at a cheaper cost.
- Photocatalysis of organic reactions by polypyridyl complexes,[53] porphyrins[54] or other dyes[55] is extensively used by organic chemists to produce materials inaccessible by classical approaches. Most of the photocatalytic dye degradation studies reported have been with Titanium dioxide as a photocatalyst. However, the major disadvantage of TiO2 that it absorbs only in the UV region since it has a band gap of around 3.2 eV. Among the different phases of TiO2, anatase form of TiO2 is mostly employed due to its higher photon absorption characteristics. It is clear that the phase composition of TiO2 has a role to play in degradation of dyes.[56]
- Light2CAT, a research and innovation project funded by the European Commission from 2012 to 2015, aimed to develop a modified TiO2 – able to absorb light in the visible spectrum (commercial TiO2 can only be activated under ultraviolet light, which is hard to achieve) – and include this modified TiO2 into concrete for the construction of concrete structures, to mitigate air quality in European cities. The absorption of visible light activates this modified TiO2, which degrades harmful pollutants via photocatalysis, such as NOX (a combination NO and NO2, in which the latter is harmful to human health), into harmless components, such as NO3-. The modified TiO2 was utilized in concrete in three separate European cities: Copenhagen and Holbæk, Denmark, and Valencia, Spain. The installation of this “self-cleaning” concrete lead to a 5-20% reduction in NOx over the course of a year.[57][58]
- Photocatalytic bioethanol production, research by Professor Linda Lawton, Robert Gordon University and her collaborators under CyanoSol funded by BBSRC.[59]
Quantification of Photocatalytic Activity[]
ISO 22197-1:2007 specifies a test method for the determination of the nitric oxide removal performance of materials that contain a photocatalyst or have superficial photocatalytic films.[60]
Specific FTIR systems are used to characterise photocatalytic activity or passivity, especially with respect to volatile organic compounds (VOCs), and representative matrices of the binders applied.[61]
Recent studies show that mass spectrometry can be a powerful tool to determine photocatalytic activity of certain materials by following the decomposition of gaseous pollutants such as nitrogen oxides or carbon dioxide [62]
See also[]
- Light harvesting materials
- Photoelectrochemical cell
- Photolysis
- Photocatalytic water splitting
- Photoredox catalysis
- Photoelectrochemical oxidation
- Photosensitizer
References[]
- ^ a b Shafiq, Iqrash; Shafique, Sumeer; Akhter, Parveen; Abbas, Ghulam; Qurashi, Ahsanulhaq; Hussain, Murid (2021-01-04). "Efficient catalyst development for deep aerobic photocatalytic oxidative desulfurization: recent advances, confines, and outlooks". Catalysis Reviews: 1–46. doi:10.1080/01614940.2020.1864859. ISSN 0161-4940. S2CID 234186125.
- ^ Eibner, Alexander (1911). "Action of Light on Pigments I". Chem-ZTG. 35: 753–755.
- ^ a b c d e f g h i j k l Coronado, Juan M.; Fresno, Fernando; Hernández-Alonso, María D.; Portela, Racquel (2013). Design of Advanced Photocatalytic Materials for Energy and Environmental Applications. London: Springer. pp. 1–5. doi:10.1007/978-1-4471-5061-9. hdl:10261/162776. ISBN 978-1-4471-5061-9.
- ^ Bruner, L.; Kozak, J. (1911). "Information on the Photocatalysis I The Light Reaction in Uranium Salt Plus Oxalic Acid Mixtures". Elktrochem Agnew P. 17: 354–360.
- ^ Landau, M. (1913). "Le Phénomène de la Photocatalyse". Compt. Rend. 156: 1894–1896.
- ^ Baly, E.C.C.; Helilbron, I.M.; Barker, W.F. (1921). "Photocatalysis. Part I. The Synthesis of Formaldehyde and Carbohydrates from Carbon Dioxide and Water". J Chem Soc. 119: 1025–1035. doi:10.1039/CT9211901025.
- ^ Goodeve, C.F.; Kitchener, J.A. (1938). "The Mechanism of Photosensitization by Solids". Transactions of the Faraday Society. 34: 902–912. doi:10.1039/tf9383400902.
- ^ Filimonov, V.N. (1964). "Photocatalytic Oxidation of Gaseous Isopropanol on ZnO + TiO2". Dokl. Akad. Nauk SSSR. 154 (4): 922–925.
- ^ Ikekawa, A.; Kamiya, M.; Fujita, Y.; Kwan, T. (1965). "On the Competition of Homogeneous and Heterogeneous Chain Terminations in Heterogeneous Photooxidation Catalysis by Zinc Oxide". Bulletin of the Chemical Society of Japan. 38: 32–36. doi:10.1246/bcsj.38.32.
- ^ Doerffler, W.; Hauffe, K. (1964). "Heterogeneous Photocatalysis I. Influence of Oxidizing and Reducing Gases on the Electrical Conductivity of Dark and Illuminated Zinc Oxide Surfaces". J Catal. 3 (2): 156–170. doi:10.1016/0021-9517(64)90123-X.
- ^ Kato, S.; Mashio, F. (1964). "Titanium Dioxide-Photocatalyzed Liquid Phase Oxidation of Tetralin". The Journal of the Society of Chemical Industry, Japan. 67 (8): 1136–1140. doi:10.1246/nikkashi1898.67.8_1136.
- ^ Formenti, M.; Julliet F., F.; Teichner SJ, S.J. (1970). "Controlled Photooxidation of Paraffins and Olefins over Anatase at Room Temperature". Comptes Rendus de l'Académie des Sciences, Série C. 270C: 138–141.
- ^ Tanaka, K.I.; Blyholde, G. (1970). "Photocatalytic and Thermal Catalytic Decomposition of Nitrous Oxide on Zinc Oxide". J. Chem. Soc. D. 18 (18): 1130. doi:10.1039/c29700001130.
- ^ Fujishima, A.; Honda, K. (1972). "Electrochemical Photolysis of Water at a Semiconductor Electrode". Nature. 238 (5358): 37–38. Bibcode:1972Natur.238...37F. doi:10.1038/238037a0. PMID 12635268. S2CID 4251015.
- ^ Nozik, A.J. (1977). "Photochemical Diodes". Appl Phys Lett. 30 (11): 567–570. Bibcode:1977ApPhL..30..567N. doi:10.1063/1.89262.
- ^ Wagner, F.T.; Somorjai, G.A. (1980). "Photocatalytic and Photoelectrochemical Hydrogen Production on Strontium Titanate Single Crystals". J Am Chem Soc. 102 (17): 5494–5502. doi:10.1021/ja00537a013.
- ^ Sakata, T.; Kawai, T. (1981). "Heterogeneous Photocatalytic Production of Hydrogen and Methane from Ethanol and Water". Chem Phys Lett. 80 (2): 341–344. Bibcode:1981CPL....80..341S. doi:10.1016/0009-2614(81)80121-2.
- ^ Chu, S.; Li, W.; Yan, Y.; Hamann, T.; Shih, I.; Wang, D.; Mi, Z. (2017). "Roadmap on Solar Water Splitting: Current Status and Future Prospects". Nano Futures. IOP Publishing Ltd. 1 (2): 022001. Bibcode:2017NanoF...1b2001C. doi:10.1088/2399-1984/aa88a1.
- ^ Wu, CH; Chang, CL (2006). "Decolorization of Reactive Red 2 by advanced oxidation processes: Comparative studies of homogeneous and heterogeneous systems". Journal of Hazardous Materials. 128 (2–3): 265–72. doi:10.1016/j.jhazmat.2005.08.013. PMID 16182444.
- ^ Peternel, IT; Koprivanac, N; Bozić, AM; Kusić, HM (2007). "Comparative study of UV/TiO2, UV/ZnO and photo-Fenton processes for the organic reactive dye degradation in aqueous solution". Journal of Hazardous Materials. 148 (1–2): 477–84. doi:10.1016/j.jhazmat.2007.02.072. PMID 17400374.
- ^ P. Cie´sla (August 2004). "Homogeneous photocatalysis by transition metal complexes in the environment". Journal of Molecular Catalysis A: Chemical 224 (2004) 17–33: 23.
- ^ Cervone, E.; Diomedi Camassei, F.; Giannini, I.; Sykora, J. (January 1979). "Photoredox behaviour of chlorocopper(II) complexes in acetonitrile: mechanism and quantum yields". Journal of Photochemistry. 11 (5): 321–332. doi:10.1016/0047-2670(79)85021-2. ISSN 0047-2670.
- ^ a b Cieśla, Paweł; Kocot, Przemysław; Mytych, Piotr; Stasicka, Zofia (2004-12-15). "Homogeneous photocatalysis by transition metal complexes in the environment". Journal of Molecular Catalysis A: Chemical. Special Issue Dedicated to Professor Jozef J. Ziolkowski on the Occasion of His 70th Birthday. 224 (1): 17–33. doi:10.1016/j.molcata.2004.08.043. ISSN 1381-1169.
- ^ Linsebigler, Amy L.; Lu, Guangquan.; Yates, John T. (1995). "Photocatalysis on TiO2 Surfaces: Principles, Mechanisms, and Selected Results". Chemical Reviews. 95 (3): 735–758. doi:10.1021/cr00035a013.
- ^ Karvinen, S.;Hirva, P;Pakkanen, T.A. (2003). "Ab initio quantum chemical studies of cluster models for doped anatase and rutile TiO2". Journal of Molecular Structure: Theochem. 626 (1–3): 271–277. doi:10.1016/S0166-1280(03)00108-8.CS1 maint: multiple names: authors list (link)
- ^ Daneshvar, N; Salari, D; Khataee, A.R (2004). "Photocatalytic degradation of azo dye acid red 14 in water on ZnO as an alternative catalyst to TiO2". Journal of Photochemistry and Photobiology A: Chemistry. 162 (2–3): 317–322. doi:10.1016/S1010-6030(03)00378-2.
- ^ a b Ibhadon, Alex; Fitzpatrick, Paul (2013-03-01). "Heterogeneous Photocatalysis: Recent Advances and Applications". Catalysts. 3 (1): 189–218. doi:10.3390/catal3010189. ISSN 2073-4344.
 Text was copied from this source, which is available under a Creative Commons Attribution 3.0 (CC BY 3.0) license.
Text was copied from this source, which is available under a Creative Commons Attribution 3.0 (CC BY 3.0) license.
- ^ a b Sandberg, Mats; Håkansson, Karl; Granberg, Hjalmar (2020-10-01). "Paper machine manufactured photocatalysts - Lateral variations". Journal of Environmental Chemical Engineering. 8 (5): 104075. doi:10.1016/j.jece.2020.104075. ISSN 2213-3437.
- ^ Nunes, Daniela; Pimentel, Ana; Branquinho, Rita; Fortunato, Elvira; Martins, Rodrigo (2021-04-16). "Metal Oxide-Based Photocatalytic Paper: A Green Alternative for Environmental Remediation". Catalysts. 11 (4): 504. doi:10.3390/catal11040504. ISSN 2073-4344.
- ^ Kudo, Akihiko; Kato, Hideki; Tsuji, Issei (2004). "Strategies for the Development of Visible-light-driven Photocatalysts for Water Splitting". Chemistry Letters. 33 (12): 1534. doi:10.1002/chin.200513248.
- ^ Shafiq, Iqrash; Hussain, Murid; Shafique, Sumeer; Rashid, Ruhma; Akhter, Parveen; Ahmed, Ashfaq; Jeon, Jong-Ki; Park, Young-Kwon (2021-06-25). "Oxidative desulfurization of refinery diesel pool fractions using LaVO4 photocatalyst". Journal of Industrial and Engineering Chemistry. 98: 283–288. doi:10.1016/j.jiec.2021.03.040. ISSN 1226-086X. S2CID 233604050.
- ^ Gholipour, Mohammad Reza; Dinh, Cao-Thang; Béland, François; Do, Trong-On (2015-04-30). "Nanocomposite heterojunctions as sunlight-driven photocatalysts for hydrogen production from water splitting". Nanoscale. 7 (18): 8187–8208. Bibcode:2015Nanos...7.8187R. doi:10.1039/C4NR07224C. ISSN 2040-3372. PMID 25804291.
- ^ Xie, Lihong; Ai, Zhuyu; Zhang, Meng; Sun, Runze; Zhao, Weirong (2016-08-30). "Enhanced Hydrogen Evolution in the Presence of Plasmonic Au-Photo-Sensitized g-C3N4 with an Extended Absorption Spectrum from 460 to 640 nm". PLOS ONE. 11 (8): e0161397. Bibcode:2016PLoSO..1161397X. doi:10.1371/journal.pone.0161397. ISSN 1932-6203. PMC 5004922. PMID 27575246.
- ^ Banić, Nemanja; Krstić, Jugoslav; Stojadinović, Stevan; Brnović, Anđela; Djordjevic, Aleksandar; Abramović, Biljana (September 2020). "Commercial TiO 2 loaded with NiO for improving photocatalytic hydrоgen prоduction in the presence оf simulated solar radiation". International Journal of Energy Research. 44 (11): 8951–8963. doi:10.1002/er.5604. ISSN 0363-907X. S2CID 225707644.
- ^ "Snapcat Photo Catalytic Oxidation with Titanium Dioxide (2005)". CaluTech UV Air. Retrieved 2006-12-05.
- ^ Kondrakov AO, Ignatev AN, Lunin VV, Frimmel FH, Braese S, Horn H (2016). "Roles of water and dissolved oxygen in photocatalytic generation of free OH radicals in aqueous TiO2 suspensions: An isotope labeling study". Applied Catalysis B: Environmental. 182: 424–430. doi:10.1016/j.apcatb.2015.09.038.
- ^ "Photocatalysis Applications of Titanium Dioxide TiO2". Titanium Information. titaniumart.com.
- ^ Kondrakov AO, Ignatev AN, Frimmel FH, Braese S, Horn H, Revelsky AI (2014). "Formation of genotoxic quinones during bisphenol A degradation by TiO2 photocatalysis and UV photolysis: A comparative study". Applied Catalysis B: Environmental. 160: 106–114. doi:10.1016/j.apcatb.2014.05.007.
- ^ Banerjee, Swagata; Dionysiou, Dionysios D.; Pillai, Suresh C. (October 2015). "Self-cleaning applications of TiO2 by photo-induced hydrophilicity and photocatalysis". Applied Catalysis B: Environmental. 176–177: 396–428. doi:10.1016/j.apcatb.2015.03.058. ISSN 0926-3373.
- ^ Shafiq, Iqrash; Hussain, Murid; Shehzad, Nasir; Maafa, Ibrahim M.; Akhter, Parveen; Amjad, Um-e-salma; Shafique, Sumeer; Razzaq, Abdul; Yang, Wenshu; Tahir, Muhammad; Russo, Nunzio (2019-08-01). "The effect of crystal facets and induced porosity on the performance of monoclinic BiVO4 for the enhanced visible-light driven photocatalytic abatement of methylene blue". Journal of Environmental Chemical Engineering. 7 (4): 103265. doi:10.1016/j.jece.2019.103265. ISSN 2213-3437. S2CID 198742844.
- ^ McCullagh C, Robertson JM, Bahnemann DW, Robertson PK (2007). "The application of TiO2 photocatalysis for disinfection of water contaminated with pathogenic micro-organisms: a review". Research on Chemical Intermediates. 33 (3–5): 359–375. doi:10.1163/156856707779238775. S2CID 94649652.
- ^ Hanaor, Dorian A. H.; Sorrell, Charles C. (2014). "Sand Supported Mixed-Phase TiO2 Photocatalysts for Water Decontamination Applications". Advanced Engineering Materials. 16 (2): 248–254. arXiv:1404.2652. Bibcode:2014arXiv1404.2652H. doi:10.1002/adem.201300259. S2CID 118571942.
- ^ Cushnie TP, Robertson PK, Officer S, Pollard PM, Prabhu R, McCullagh C, Robertson JM (2010). "Photobactericidal effects of TiO2 thin films at low temperature". Journal of Photochemistry and Photobiology A: Chemistry. 216 (2–3): 290–294. doi:10.1016/j.jphotochem.2010.06.027.
- ^ Kostedt IV, William L.; Jack Drwiega; David W. Mazyck; Seung-Woo Lee; Wolfgang Sigmund; Chang-Yu Wu; Paul Chadik (2005). "Magnetically agitated photocatalytic reactor for photocatalytic oxidation of aqueous phase organic pollutants". Environmental Science & Technology. American Chemical Society. 39 (20): 8052–8056. Bibcode:2005EnST...39.8052K. doi:10.1021/es0508121. PMID 16295874.
- ^ Tan, S. S.; L. Zou; E. Hu (2006). "Photocatalytic reduction of carbon dioxide into gaseous hydrocarbon using TiO2 pellets". Catalysis Today. 115 (1–4): 269–273. doi:10.1016/j.cattod.2006.02.057.
- ^ Yao, Y. Yao; G. Li; S. Ciston; R. M. Lueptow; K. Gray (2008). "Photoreactive TiO2/Carbon Nanotube Composites: Synthesis and Reactivity". Environmental Science & Technology. American Chemical Society. 42 (13): 4952–4957. Bibcode:2008EnST...42.4952Y. doi:10.1021/es800191n. PMID 18678032.
- ^ Linsebigler, A. L.; G. Lu; J.T. Yates (1995). "Photocatalysis on TiO2 Surfaces: Principles, Mechanisms, and Selected Results". Chemical Reviews. 95 (3): 735–758. doi:10.1021/cr00035a013.
- ^ "New visible light photocatalyst kills bacteria, even after light turned off". ScienceDaily.
- ^ Lu, Yawei; Chen, Ting; Chen, Xianfu; Qiu, Minghui; Fan, Yiqun (September 2016). "Fabrication of TiO2-doped ZrO2 nanofiltration membranes by using a modified colloidal sol-gel process and its application in simulative radioactive effluent". Journal of Membrane Science. 514: 476–486. doi:10.1016/j.memsci.2016.04.074.
- ^ Bortot Coelho, Fabrício; Gionco, Chiara; Paganini, Maria; Calza, Paola; Magnacca, Giuliana (3 April 2019). "Control of Membrane Fouling in Organics Filtration Using Ce-Doped Zirconia and Visible Light". Nanomaterials. 9 (4): 534. doi:10.3390/nano9040534. PMC 6523972. PMID 30987140.
- ^ Bortot Coelho, Fabrício Eduardo; Candelario, Victor M.; Araújo, Estêvão Magno Rodrigues; Miranda, Tânia Lúcia Santos; Magnacca, Giuliana (18 April 2020). "Photocatalytic Reduction of Cr(VI) in the Presence of Humic Acid Using Immobilized Ce–ZrO2 under Visible Light". Nanomaterials. 10 (4): 779. doi:10.3390/nano10040779. PMC 7221772. PMID 32325680.
- ^ ROUZAFZAY, F.; SHIDPOUR, R. (2020). "Graphene@ZnO nanocompound for short-time water treatment under sun-simulated irradiation: Effect of shear exfoliation of graphene using kitchen blender on photocatalytic degradation". Alloys and Compounds. 829: 154614. doi:10.1016/J.JALLCOM.2020.154614. S2CID 216233251.
- ^ Stephenson, Corey; Yoon, Tehshik; MacMillan, David W. C. (2018-04-02). Visible Light Photocatalysis in Organic Chemistry. doi:10.1002/9783527674145. ISBN 9783527674145.
- ^ Barona-Castaño, Juan C.; Carmona-Vargas, Christian C.; Brocksom, Timothy J.; De Oliveira, Kleber T. (March 2016). "Porphyrins as Catalysts in Scalable Organic Reactions". Molecules. 21 (3): 310. doi:10.3390/molecules21030310. PMC 6273917. PMID 27005601.
- ^ Sirbu, Dumitru; Woodford, Owen J.; Benniston, Andrew C.; Harriman, Anthony (2018-06-13). "Photocatalysis and self-catalyzed photobleaching with covalently-linked chromophore-quencher conjugates built around BOPHY". Photochemical & Photobiological Sciences. 17 (6): 750–762. doi:10.1039/C8PP00162F. ISSN 1474-9092. PMID 29717745.
- ^ Viswanathan, Balasubramanian (December 2017). "Photocatalytic Degradation of Dyes: An Overview". Current Catalysis, 2018, 7, 000-000: 3.
- ^ Mathiesen, D. (2012). "Final Report Summary - LIGHT2CAT (Visible LIGHT Active PhotoCATalytic Concretes for Air pollution Treatment)". European Commission.
- ^ Light2CAT (2015). "Light2CAT Visible Light Active PhotoCATalytic Concretes for Air Pollution Treatment [YouTube Video]". YouTube. Archived from the original on 2021-12-13.
- ^ March 31, Posted on; 2017 (2017-03-31). "Major funding granted to CyanoSol, Robert Gordon University". Algal Solutions For Local Energy Economy (ASLEE). Retrieved 2021-06-25.CS1 maint: numeric names: authors list (link)
- ^ "ISO 22197-1:2007". ISO.
- ^ "Unique Gas Analyser Helps to Characterize Photoactive Pigments | CoatingsPro Magazine". www.coatingspromag.com.
- ^ Nuño, Manuel (2014). "Study of solid/gas phase photocatalytic reactions by electron ionization mass spectrometry" (PDF). Journal of Mass Spectrometry. 49 (8): 716–726. Bibcode:2014JMSp...49..716N. doi:10.1002/jms.3396. PMID 25044899.
- Photochemistry
- Catalysis
![{\displaystyle {\ce {[Cu^{II}Lx]^2 ->[{hν(LMCT)}][{}] [Cu^{I}Lx-1]^+ +L{.}^{+}}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/77f51faa55a9c82334265edbcca915fbb076f65a)
![{\displaystyle {\ce {[CuClx]^{2-x}->[{hν(LMCT)}][{}] Cl. + [CuCl{x-1}]^{2-x}}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/db87a77f03ced980afc510a81c260baaecce1d2a)

![{\displaystyle {\ce {TiO2 ->[{hv}][{}] e-(TiO2) + h+(TiO2)}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/76a2fcc031e4a874388c39b885ad1a0735806cf0)
