Piceol
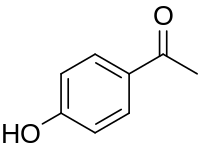 Chemical structure of piceol
| |
| Names | |
|---|---|
| Preferred IUPAC name
1-(4-Hydroxyphenyl)ethan-1-one | |
| Other names
1-(4-Hydroxyphenyl)ethanone
4-Hydroxy acetophenone 4'-Hydroxy acetophenone p-Hydroxyacetophenone | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.002.548 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| Properties | |
| C8H8O2 | |
| Molar mass | 136.150 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Piceol is a phenolic compound found in the needles and in mycorrhizal roots of Norway spruces (Picea abies).[1][2] Picein is the glucoside of piceol.[3]
Uses[]
Piceol is used in the synthesis of several pharmaceutical drugs including octopamine, sotalol, bamethan, and dyclonine.[citation needed]
Piceol can be used to make acetaminophen by oxime formation with hydroxylamine and subsequent Beckmann rearrangement in acid.[4]
Anticonvulsants are also possible by Mannich reaction:[5]
Metabolism[]
Diprenylated derivatives of piceol can be isolated from .[6]
4-Hydroxyacetophenone monooxygenase is an enzyme that transforms piceol into O-acetylhydroquinone. This enzyme is found in Pseudomonas fluorescens.
See also[]
References[]
- ^ Løkke, H. (1990). "Picein and piceol concentrations in Norway spruce". Ecotoxicology and Environmental Safety. 19 (3): 301–9. doi:10.1016/0147-6513(90)90032-z. PMID 2364913.
- ^ Münzenberger, Babette; Heilemann, Jürgen; Strack, Dieter; Kottke, Ingrid; Oberwinkler, Franz (1990). "Phenolics of mycorrhizas and non-mycorrhizal roots of Norway spruce". Planta. 182 (1): 142–8. doi:10.1007/BF00239996. PMID 24197010.
- ^ Løkke, Hans (1990). "Picein and piceol concentrations in Norway spruce". Ecotoxicology and Environmental Safety. 19 (3): 301–309. doi:10.1016/0147-6513(90)90032-Z. PMID 2364913.
- ^ U.S. Patent 4,524,217
- ^ Keshari, Amit K.; Tewari, Aseem; Verma, Shweta S.; Saraf, Shailendra K. (2017). "Novel Mannich-bases as Potential Anticonvulsants: Syntheses, Characterization and Biological Evaluation". Central Nervous System Agents in Medicinal Chemistry. 17 (3). doi:10.2174/1871524917666170717113524. ISSN 1871-5249.
- ^ Sigstad, Elizabeth; Catalán, César A.N.; Diaz, Jesús G.; Herz, Werner (1993). "Diprenylated derivatives of p-hydroxyacetophenone from Ophryosporus macrodon". Phytochemistry. 33: 165–169. doi:10.1016/0031-9422(93)85415-N.
Categories:
- Aromatic ketones
- Phenols