Polyol
A polyol is an organic compound containing multiple hydroxyl (HO− radical) groups. The term "polyol" can have slightly different meanings depending on whether it is being used in the field of food science or that of polymer chemistry. Polyols containing two, three and four hydroxyl groups are a diol, triol, tetrol and so on.
Classification[]
Low molecular weight polyols[]

Low molecular weight polyols are widely used in polymer chemistry, where they function as crosslinking agents. Alkyd resins for example are used in paints and in molds for casting. They are the dominant resin or "binder" in most commercial "oil-based" coatings. Approximately 200,000 tons of alkyd resins are produced each year. They are based on linking reactive monomers with through ester formation. Polyols used in the production of commercial alkyd resins are glycerol, trimethylolpropane, and pentaerythritol.[1]
| Low molecular weight polyols |
Pentaerythritol |
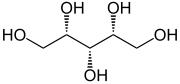 |
Sugar alcohols[]
Sugar alcohols, a class of low molecular weight polyols, are commonly obtained by hydrogenation of sugars. They have the formula (CHOH)nH2, where n = 4–6.[2]
Sugar alcohols are added to foods because of their lower caloric content than sugars; however, they are also, in general, less sweet, and are often combined with high-intensity sweeteners. They are also added to chewing gum because they are not broken down by bacteria in the mouth or metabolized to acids, and thus do not contribute to tooth decay. Maltitol, sorbitol, xylitol, erythritol, and isomalt are common sugar alcohols.
Polymeric polyols[]
| Polymeric polyols |
 Polyether polyol (The oxygen atoms of the ether linkages are shown in blue.) |
 Polyester polyol (The oxygen and carbon atoms of the ester groups are shown in blue.) |
The term polyol is used for polyether polyols and polyester polyols. A typical example is polyethylene oxide or polyethylene glycol (PEG) and polypropylene glycol (PPG). Polytetrahydrofuran or PTMEG is another polyol molecule. Polyols are reacted with diisocyanates to produce polyurethanes. Polyurethanes are used to make flexible foam for mattresses and seating, rigid foam insulation for refrigerators and freezers, elastomeric shoe soles, fibers (e.g. Spandex), coatings, sealants and adhesives.
The term "polyol" is also attributed to other molecules containing hydroxyl groups. For instance, polyvinyl alcohol is (CH2CHOH)n with n hydroxyl groups where n can be in the thousands. Cellulose is a polymer with many hydroxyl groups, but it is not referred to as a polyol.
Properties[]
Since the generic term polyol is only derived from chemical nomenclature and just indicates the presence of several hydroxyl groups, no common properties can be assigned to all polyols. However, polyols are usually highly viscous (when polymeric) to solid (when low-molecular weight) at room temperature due to hydrogen bonding.
See also[]
References[]
- ^ Frank N. Jones. "Alkyd Resins". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a01_409.
- ^ Hubert Schiweck, Albert Bär, Roland Vogel, Eugen Schwarz, Markwart Kunz, Cécile Dusautois, Alexandre Clement, Caterine Lefranc, Bernd Lüssem, Matthias Moser, Siegfried Peters (2012). "Sugar Alcohols". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a25_413.pub3. ISBN 978-3527306732.CS1 maint: uses authors parameter (link)
External links[]
 Media related to Polyols at Wikimedia Commons
Media related to Polyols at Wikimedia Commons
- Polyols
- Sugar substitutes
- Organic polymers
- Commodity chemicals