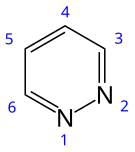
Pyridazine
| |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Pyridazine[1] | |||
| Systematic IUPAC name
1,2-Diazabenzene | |||
| Other names
1,2-Diazine
Orthodiazine Oizine | |||
| Identifiers | |||
| |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.005.478 | ||
| EC Number |
| ||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| Properties | |||
| C4H4N2 | |||
| Molar mass | 80.090 g·mol−1 | ||
| Appearance | Colorless liquid | ||
| Density | 1.107 g/cm3 | ||
| Melting point | −8 °C (18 °F; 265 K) | ||
| Boiling point | 208 °C (406 °F; 481 K) | ||
| miscible | |||
| Solubility | miscible in dioxane, ethanol soluble in benzene, diethyl ether negligible in cyclohexane, ligroin | ||
Refractive index (nD)
|
1.52311 (23.5 °C) | ||
| Thermochemistry | |||
Std enthalpy of
formation (ΔfH⦵298) |
224.9 kJ/mol | ||
| Hazards | |||
| Flash point | 85 °C (185 °F; 358 K) | ||
| Related compounds | |||
Related compounds
|
pyridine, pyrimidine, pyrazine | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Pyridazine is a heterocyclic organic compound with the molecular formula (CH)4N2. It contains a six-membered ring with two adjacent nitrogen atoms, and is aromatic.[2] It is a colorless liquid with a boiling point of 208 °C. It is isomeric with two other (CH)4N2 rings, pyrimidine and pyrazine.
Occurrence[]
Pyridazines are rare in nature, possibly reflecting the scarcity of naturally occurring hydrazines, common building blocks for the synthesis of these heterocycles. The pyridazine structure is a popular pharmacophore which is found within a number of herbicides such as , and . It is also found within the structure of several drugs such as cefozopran, cadralazine, minaprine, pipofezine, and hydralazine.
Synthesis[]
In the course of his classic investigation on the Fischer indole synthesis, Emil Fischer prepared the first pyridazine via the condensation of phenylhydrazine and levulinic acid.[3] The parent heterocycle was first prepared by oxidation of benzocinnoline to the pyridazinetetracarboxylic acid followed by decarboxylation. A better route to this otherwise esoteric compound starts with the maleic hydrazide. These heterocycles are often prepared via condensation of 1,4-diketones or 4-ketoacids with hydrazines.[4]
References[]
- ^ "Front Matter". Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 141. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- ^ Gumus, S. (2011). "A computational study on substituted diazabenzenes" (PDF). Turk J Chem. 35: 803–808. Archived from the original (PDF) on 2016-03-03. Retrieved 2014-04-10.
- ^ Fischer, E. (1886). "Indole aus Phenylhydrazin". Justus Liebigs Annalen der Chemie. 236 (1–2): 126–151. doi:10.1002/jlac.18862360107.
- ^ Tišler, M.; Stanovnik, B. (1968). "Pyridazines". Advances in Heterocyclic Chemistry. 9: 211–320. doi:10.1016/S0065-2725(08)60374-8. ISBN 9780120206094.
- Pyridazines
- Simple aromatic rings