RALA
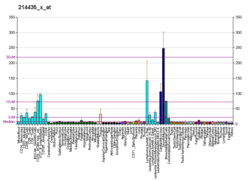
Ras-related protein Ral-A (RalA) is a protein that in humans is encoded by the RALA gene on chromosome 7.[5][6] This protein is one of two paralogs of the Ral protein, the other being RalB, and part of the Ras GTPase family.[7] RalA functions as a molecular switch to activate a number of biological processes, majorly cell division and transport, via signaling pathways.[7][8][9] Its biological role thus implicates it in many cancers.[9]
Structure[]
The Ral isoforms share an 80% overall match in amino acid sequence and 100% match in their effector-binding region. The two isoforms mainly differ in the C-terminal hypervariable region, which contains multiple sites for post-translational modification, leading to diverging subcellular localization and biological function. For example, phosphorylation of Serine 194 on RalA by the kinase Aurora A results in the relocation of RalA to the inner mitochondrial membrane, where RalA helps carry out mitochondrial fission; whereas phosphorylation of Serine 198 on RalB by the kinase PKC results in the relocation of RalB to other internal membranes and activation of its tumorigenic function.[9]
Function[]
RalA is one of two proteins in the Ral family, which is itself a subfamily within the Ras family of small GTPases.[7] As a Ras GTPase, RalA functions as a molecular switch that becomes active when bound to GTP and inactive when bound to GDP. RalA can be activated by RalGEFs and, in turn, activate effectors in signal transduction pathways leading to biological outcomes.[7][8] For instance, RalA interacts with two components of the exocyst, Exo84 and Sec5, to promote autophagosome assembly, secretory vesicle trafficking, and tethering. Other downstream functions include exocytosis, receptor-mediated endocytosis, tight junction biogenesis, filopodia formation, mitochondrial fission, and cytokinesis.[7][9][10] Ral-mediated exocytosis is also involved such biological processes as platelet activation, immune cell functions, neuronal plasticity, and regulation of insulin action.[11]
While the above functions appear to be shared between the two Ral isoforms, their differential subcellular localizations result in their differing involvement in certain biological processes. In particular, RalA is more involved in anchorage-independent cell growth, vesicle trafficking, and cytoskeletal organization.[8][12] Moreover, RalA specifically interacts with Exo84 and Sec5 to regulate transport of membrane proteins in polarized epithelial cells and GLUT4 to the plasma membrane, as well as mitochondrial fission for cell division.[7]
Clinical significance[]
Ral proteins have been associated with the progression of several cancers, including bladder cancer and prostate cancer.[9] Though the exact mechanisms remain unclear, studies reveal that RalA promotes anchorage-independent growth in cancer cells.[8] As a result, inhibition of RalA inhibits cancer initiation.[9]
Due to its exocytotic role in platelets, immune cells, neurons, and insulin regulation, downregulation of Ral may lead to pathological conditions such as thrombosis and metabolic syndrome. In chronic thromboembolic pulmonary hypertension patients, Ral GTPases have been observed to be highly active in their platelets.[11]
Interactions[]
RalA has been shown to interact with:
References[]
- ^ a b c GRCh38: Ensembl release 89: ENSG00000006451 - Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000008859 - Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ Rousseau-Merck MF, Bernheim A, Chardin P, Miglierina R, Tavitian A, Berger R (Jun 1988). "The ras-related ral gene maps to chromosome 7p15-22". Human Genetics. 79 (2): 132–6. doi:10.1007/BF00280551. PMID 3292391. S2CID 24522661.
- ^ "Entrez Gene: RALA v-ral simian leukemia viral oncogene homolog A (ras related)".
- ^ a b c d e f g h Simicek M, Lievens S, Laga M, Guzenko D, Aushev VN, Kalev P, Baietti MF, Strelkov SV, Gevaert K, Tavernier J, Sablina AA (Oct 2013). "The deubiquitylase USP33 discriminates between RALB functions in autophagy and innate immune response". Nature Cell Biology. 15 (10): 1220–30. doi:10.1038/ncb2847. PMID 24056301. S2CID 205287526.
- ^ a b c d Tecleab A, Zhang X, Sebti SM (Nov 2014). "Ral GTPase down-regulation stabilizes and reactivates p53 to inhibit malignant transformation". The Journal of Biological Chemistry. 289 (45): 31296–309. doi:10.1074/jbc.M114.565796. PMC 4223330. PMID 25210032.
- ^ a b c d e f g Kashatus DF (Sep 2013). "Ral GTPases in tumorigenesis: emerging from the shadows". Experimental Cell Research. 319 (15): 2337–42. doi:10.1016/j.yexcr.2013.06.020. PMC 4270277. PMID 23830877.
- ^ Hazelett CC, Sheff D, Yeaman C (Dec 2011). "RalA and RalB differentially regulate development of epithelial tight junctions". Molecular Biology of the Cell. 22 (24): 4787–800. doi:10.1091/mbc.E11-07-0657. PMC 3237622. PMID 22013078.
- ^ a b Shirakawa R, Horiuchi H (May 2015). "Ral GTPases: crucial mediators of exocytosis and tumourigenesis". Journal of Biochemistry. 157 (5): 285–99. doi:10.1093/jb/mvv029. PMID 25796063.
- ^ Jeon H, Zheng LT, Lee S, Lee WH, Park N, Park JY, Heo WD, Lee MS, Suk K (Aug 2011). "Comparative analysis of the role of small G proteins in cell migration and cell death: cytoprotective and promigratory effects of RalA". Experimental Cell Research. 317 (14): 2007–18. doi:10.1016/j.yexcr.2011.05.021. PMID 21645515.
- ^ Ohta Y, Suzuki N, Nakamura S, Hartwig JH, Stossel TP (Mar 1999). "The small GTPase RalA targets filamin to induce filopodia". Proceedings of the National Academy of Sciences of the United States of America. 96 (5): 2122–8. doi:10.1073/pnas.96.5.2122. PMC 26747. PMID 10051605.
- ^ Luo JQ, Liu X, Hammond SM, Colley WC, Feig LA, Frohman MA, Morris AJ, Foster DA (Jun 1997). "RalA interacts directly with the Arf-responsive, PIP2-dependent phospholipase D1". Biochemical and Biophysical Research Communications. 235 (3): 854–9. doi:10.1006/bbrc.1997.6793. PMID 9207251.
- ^ Kim JH, Lee SD, Han JM, Lee TG, Kim Y, Park JB, Lambeth JD, Suh PG, Ryu SH (Jul 1998). "Activation of phospholipase D1 by direct interaction with ADP-ribosylation factor 1 and RalA". FEBS Letters. 430 (3): 231–5. doi:10.1016/S0014-5793(98)00661-9. PMID 9688545. S2CID 36075513.
- ^ Moskalenko S, Tong C, Rosse C, Mirey G, Formstecher E, Daviet L, Camonis J, White MA (Dec 2003). "Ral GTPases regulate exocyst assembly through dual subunit interactions". The Journal of Biological Chemistry. 278 (51): 51743–8. doi:10.1074/jbc.M308702200. PMID 14525976.
- ^ Jullien-Flores V, Dorseuil O, Romero F, Letourneur F, Saragosti S, Berger R, Tavitian A, Gacon G, Camonis JH (Sep 1995). "Bridging Ral GTPase to Rho pathways. RLIP76, a Ral effector with CDC42/Rac GTPase-activating protein activity". The Journal of Biological Chemistry. 270 (38): 22473–7. doi:10.1074/jbc.270.38.22473. PMID 7673236.
- ^ Cantor SB, Urano T, Feig LA (Aug 1995). "Identification and characterization of Ral-binding protein 1, a potential downstream target of Ral GTPases". Molecular and Cellular Biology. 15 (8): 4578–84. doi:10.1128/mcb.15.8.4578. PMC 230698. PMID 7623849.
- ^ Ikeda M, Ishida O, Hinoi T, Kishida S, Kikuchi A (Jan 1998). "Identification and characterization of a novel protein interacting with Ral-binding protein 1, a putative effector protein of Ral". The Journal of Biological Chemistry. 273 (2): 814–21. doi:10.1074/jbc.273.2.814. PMID 9422736.
Further reading[]
- Kinsella BT, Erdman RA, Maltese WA (May 1991). "Carboxyl-terminal isoprenylation of ras-related GTP-binding proteins encoded by rac1, rac2, and ralA". The Journal of Biological Chemistry. 266 (15): 9786–94. doi:10.1016/S0021-9258(18)92889-9. PMID 1903399.
- Polakis PG, Weber RF, Nevins B, Didsbury JR, Evans T, Snyderman R (Oct 1989). "Identification of the ral and rac1 gene products, low molecular mass GTP-binding proteins from human platelets". The Journal of Biological Chemistry. 264 (28): 16383–9. doi:10.1016/S0021-9258(19)84717-8. PMID 2550440.
- Chardin P, Tavitian A (Jun 1989). "Coding sequences of human ralA and ralB cDNAs". Nucleic Acids Research. 17 (11): 4380. doi:10.1093/nar/17.11.4380. PMC 317954. PMID 2662142.
- Cantor SB, Urano T, Feig LA (Aug 1995). "Identification and characterization of Ral-binding protein 1, a potential downstream target of Ral GTPases". Molecular and Cellular Biology. 15 (8): 4578–84. doi:10.1128/mcb.15.8.4578. PMC 230698. PMID 7623849.
- Jullien-Flores V, Dorseuil O, Romero F, Letourneur F, Saragosti S, Berger R, Tavitian A, Gacon G, Camonis JH (Sep 1995). "Bridging Ral GTPase to Rho pathways. RLIP76, a Ral effector with CDC42/Rac GTPase-activating protein activity". The Journal of Biological Chemistry. 270 (38): 22473–7. doi:10.1074/jbc.270.38.22473. PMID 7673236.
- Wang KL, Khan MT, Roufogalis BD (Jun 1997). "Identification and characterization of a calmodulin-binding domain in Ral-A, a Ras-related GTP-binding protein purified from human erythrocyte membrane". The Journal of Biological Chemistry. 272 (25): 16002–9. doi:10.1074/jbc.272.25.16002. PMID 9188503.
- Luo JQ, Liu X, Hammond SM, Colley WC, Feig LA, Frohman MA, Morris AJ, Foster DA (Jun 1997). "RalA interacts directly with the Arf-responsive, PIP2-dependent phospholipase D1". Biochemical and Biophysical Research Communications. 235 (3): 854–9. doi:10.1006/bbrc.1997.6793. PMID 9207251.
- Ikeda M, Ishida O, Hinoi T, Kishida S, Kikuchi A (Jan 1998). "Identification and characterization of a novel protein interacting with Ral-binding protein 1, a putative effector protein of Ral". The Journal of Biological Chemistry. 273 (2): 814–21. doi:10.1074/jbc.273.2.814. PMID 9422736.
- Vavvas D, Li X, Avruch J, Zhang XF (Mar 1998). "Identification of Nore1 as a potential Ras effector". The Journal of Biological Chemistry. 273 (10): 5439–42. doi:10.1074/jbc.273.10.5439. PMID 9488663.
- Kim JH, Lee SD, Han JM, Lee TG, Kim Y, Park JB, Lambeth JD, Suh PG, Ryu SH (Jul 1998). "Activation of phospholipase D1 by direct interaction with ADP-ribosylation factor 1 and RalA". FEBS Letters. 430 (3): 231–5. doi:10.1016/S0014-5793(98)00661-9. PMID 9688545. S2CID 36075513.
- Ohta Y, Suzuki N, Nakamura S, Hartwig JH, Stossel TP (Mar 1999). "The small GTPase RalA targets filamin to induce filopodia". Proceedings of the National Academy of Sciences of the United States of America. 96 (5): 2122–8. doi:10.1073/pnas.96.5.2122. PMC 26747. PMID 10051605.
- Wang KL, Roufogalis BD (May 1999). "Ca2+/calmodulin stimulates GTP binding to the ras-related protein ral-A". The Journal of Biological Chemistry. 274 (21): 14525–8. doi:10.1074/jbc.274.21.14525. PMID 10329639.
- Suzuki J, Yamazaki Y, Li G, Kaziro Y, Koide H, Guang L (Jul 2000). "Involvement of Ras and Ral in chemotactic migration of skeletal myoblasts". Molecular and Cellular Biology. 20 (13): 4658–65. doi:10.1128/MCB.20.13.4658-4665.2000. PMC 85875. PMID 10848592.
- de Bruyn KM, de Rooij J, Wolthuis RM, Rehmann H, Wesenbeek J, Cool RH, Wittinghofer AH, Bos JL (Sep 2000). "RalGEF2, a pleckstrin homology domain containing guanine nucleotide exchange factor for Ral". The Journal of Biological Chemistry. 275 (38): 29761–6. doi:10.1074/jbc.M001160200. PMID 10889189.
- Brymora A, Valova VA, Larsen MR, Roufogalis BD, Robinson PJ (Aug 2001). "The brain exocyst complex interacts with RalA in a GTP-dependent manner: identification of a novel mammalian Sec3 gene and a second Sec15 gene". The Journal of Biological Chemistry. 276 (32): 29792–7. doi:10.1074/jbc.C100320200. PMID 11406615.
- Sugihara K, Asano S, Tanaka K, Iwamatsu A, Okawa K, Ohta Y (Jan 2002). "The exocyst complex binds the small GTPase RalA to mediate filopodia formation". Nature Cell Biology. 4 (1): 73–8. doi:10.1038/ncb720. PMID 11744922. S2CID 9528945.
- Clough RR, Sidhu RS, Bhullar RP (Aug 2002). "Calmodulin binds RalA and RalB and is required for the thrombin-induced activation of Ral in human platelets". The Journal of Biological Chemistry. 277 (32): 28972–80. doi:10.1074/jbc.M201504200. PMID 12034722.
- Xu L, Frankel P, Jackson D, Rotunda T, Boshans RL, D'Souza-Schorey C, Foster DA (Jan 2003). "Elevated phospholipase D activity in H-Ras- but not K-Ras-transformed cells by the synergistic action of RalA and ARF6". Molecular and Cellular Biology. 23 (2): 645–54. doi:10.1128/MCB.23.2.645-654.2003. PMC 151535. PMID 12509462.
External links[]
- Genes on human chromosome 7