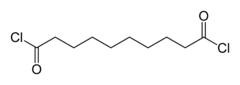
Sebacoyl chloride
| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
Decanedioyl dichloride | |
| Other names
Sebacoyl dichloride
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.003.495 |
| EC Number |
|
| MeSH | C061659 |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C10H16Cl2O2 | |
| Molar mass | 239.14 g/mol |
| Density | 1.12 g cm−3 |
| Melting point | −2.5 °C (27.5 °F; 270.6 K) |
| Boiling point | 220 °C (428 °F; 493 K) |
| Hazards | |
| Safety data sheet (SDS) | External MSDS |
| NFPA 704 (fire diamond) | 
3
0
2 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Sebacoyl chloride (or sebacoyl dichloride) is a di-acyl chloride, with formula (CH2)8(COCl)2. A colorless oily liquid with a pungent odor, it is soluble in hydrocarbons and ethers. Sebacoyl chloride is corrosive; like all acyl chlorides, it hydrolyzes, evolving hydrogen chloride. It is less susceptible to hydrolysis though than shorter chain aliphatic acyl chlorides.[1]
Preparation[]
Sebacoyl chloride can be prepared by reacting sebacic acid with an excess of thionyl chloride. Residual thionyl chloride can be removed by distillation.[2]
Use[]
Sebacoyl chloride can be polymerized with hexamethylenediamine yielding nylon-6,10.[3]
See also[]
References[]
- ^ Morgan, Paul W.; Kwolek, Stephanie L. (April 1959). "The nylon rope trick: Demonstration of condensation polymerization". Journal of Chemical Education. 36 (4): 182. Bibcode:1959JChEd..36..182M. doi:10.1021/ed036p182.
- ^ Erdmann, L.; Uhrich, K.E. (October 2000). "Synthesis and degradation characteristics of salicylic acid-derived poly(anhydride-esters)". Biomaterials. 21 (19): 1941–1946. doi:10.1016/S0142-9612(00)00073-9. PMID 10941915.
- ^ Enkelmann, Volker; Wegner, Gerhard (1976-11-01). "Mechanism of interfacial polycondensation and the direct synthesis of stable polyamide membranes". Die Makromolekulare Chemie. 177 (11): 3177–3189. doi:10.1002/macp.1976.021771106. ISSN 0025-116X.
Categories:
- Acyl chlorides
- Monomers