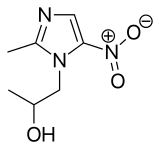
Secnidazole
 | |
| Clinical data | |
|---|---|
| Trade names | Solosec |
| Other names | PM 185184, RP 14539 |
| AHFS/Drugs.com | International Drug Names |
| License data |
|
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.020.123 |
| Chemical and physical data | |
| Formula | C7H11N3O3 |
| Molar mass | 185.183 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Secnidazole (trade names Flagentyl, Sindose, Secnil, Solosec) is a nitroimidazole anti-infective. Effectiveness in the treatment of dientamoebiasis has been reported.[1] It has also been tested against Atopobium vaginae.[2]
In the United States, secnidazole is approved for the treatment of bacterial vaginosis and trichomoniasis in adult women.[3]
References[]
- ^ Girginkardeşler N, Coşkun S, Cüneyt Balcioğlu I, Ertan P, Ok UZ (February 2003). "Dientamoeba fragilis, a neglected cause of diarrhea, successfully treated with secnidazole". Clinical Microbiology and Infection. 9 (2): 110–3. doi:10.1046/j.1469-0691.2003.00504.x. PMID 12588330.
- ^ De Backer E, Dubreuil L, Brauman M, Acar J, Vaneechoutte M (May 2010). "In vitro activity of secnidazole against Atopobium vaginae, an anaerobic pathogen involved in bacterial vaginosis". Clinical Microbiology and Infection. 16 (5): 470–2. doi:10.1111/j.1469-0691.2009.02852.x. PMID 19548924.
- ^ "FDA Approves Symbiomix Therapeutics' Solosec (secnidazole) Oral Granules for the Treatment of Bacterial Vaginosis in Adult Women" (Press release). Symbiomix Therapeutics.
Further reading[]
- Gillis JC, Wiseman LR (April 1996). "Secnidazole. A review of its antimicrobial activity, pharmacokinetic properties and therapeutic use in the management of protozoal infections and bacterial vaginosis". Drugs. 51 (4): 621–38. doi:10.2165/00003495-199651040-00007. PMID 8706597.
Categories:
- Nitroimidazole antibiotics
- Antiprotozoal agents
- Antiinfective agent stubs