Sulfur isotope biogeochemistry
Sulfur isotope biogeochemistry is the study of the distribution of sulfur isotopes in biological and geological materials. In addition to its common isotope, 32S, sulfur has three rare stable isotopes: 34S, 36S, and 33S. The distribution of these isotopes in the environment is controlled by many biochemical and physical processes, including biological metabolisms, mineral formation processes, and atmospheric chemistry. Measuring the abundance of sulfur stable isotopes in natural materials, like bacterial cultures, minerals, or seawater, can reveal information about these processes both in the modern environment and over Earth history.[1]
Background[]
Natural abundance of sulfur isotopes[]
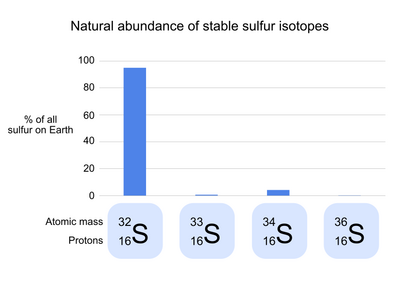
Sulfur has 24 known isotopes,[2] 4 of which are stable (meaning that they do not undergo radioactive decay).[3] 32S, the common isotope of sulfur, makes up 95.0% of the natural sulfur on Earth.[2] In the atomic symbol of 32S, the number 32 refers to the mass of each sulfur atom in Daltons, the result of the 16 protons and 16 neutrons of 1 Dalton each that make up the sulfur nucleus. The three rare stable isotopes of sulfur are 34S (4.2% of natural sulfur), 33S (0.75%), and 36S (0.015%).[4] These isotopes differ from 32S in the number of neutrons in each atom, but not the number of protons or electrons; as a result, each isotope has a slightly different mass, but has nearly identical chemical properties.[3]
Physical chemistry[]
Small differences in mass between stable isotopes of the same element can lead to a phenomenon called an "isotope effect," where heavier or lighter isotopes are preferentially incorporated into different natural materials depending on the materials' chemical composition or physical state.[5] Isotope effects are divided into two main groups: kinetic isotope effects and equilibrium isotope effects.[5] A kinetic isotope effect occurs when a reaction is irreversible, meaning that the reaction only proceeds in the direction from reactants to products.[3][5] Kinetic isotope effects cause isotopic fractionation—meaning that they affect the isotopic composition of reactant and product compounds—because the mass differences between stable isotopes can affect the rate of chemical reactions.[5] It takes more energy to reach the transition state of a reaction if the compound has bonds with a heavier isotope, which causes the compound with heavier isotopes to react more slowly.[5] Normal kinetic isotope effects cause the lighter isotope (or isotopes) to be preferentially included in a reaction's product.[5] The products are then said to be "depleted" in the heavy isotope relative to the reactant.[3] Rarely, inverse kinetic isotope effects may occur, where the heavier isotope is preferentially included in a reaction's product.[5][6]
Equilibrium isotope effects cause fractionation because it is more chemically favorable for heavy isotopes to take part in stronger bonds.[5] An equilibrium isotope effect occurs when a reaction is at equilibrium, meaning that the reaction is able to occur in both directions simultaneously.[3] When a reaction is at equilibrium, heavy isotopes will preferentially accumulate where they can form the strongest bonds.[3] For example, when the water in a sealed, half-full bottle is in equilibrium with the vapor above it, the heavier isotopes 2H and 18O will accumulate in the liquid, where they form stronger bonds, while the lighter isotopes 1H and 16O will accumulate in the vapor.[7] The liquid is then said to be "enriched" in the heavy isotope relative to the vapor.[3]
Calculations[]
Delta notation[]
Differences in the abundance of stable isotopes among natural materials are usually very small (natural differences in the ratio of rare to common isotope are almost always below 0.1%, and sometimes much smaller).[5] Nevertheless, these very small differences can record meaningful biological and geological processes. To facilitate comparison of these small but meaningful differences, isotope abundances in natural materials are often reported relative to isotope abundances in designated standards.[3][5] The convention for reporting the measured difference between a sample and a standard is called "delta notation." For example, imagine an element X for which we wish to compare the rare, heavy stable isotope with atomic mass A (AX) to the light, common isotope with atomic mass B (BX). The abundance of AX and BX in any given material is reported with the notation δAX. δAX for the sample material is calculated as follows:[5]
AR = (total amount of AX)/(total amount of BX)
δAXsample = (ARsample - ARstandard)/ARstandard
δ values are most commonly reported in parts per thousand, commonly referred to in isotope chemistry as per mille and represented by the symbol ‰. To report δ values in per mille, the δ value as calculated above should be multiplied by 1000:
δAXsample (‰) = ((ARsample - ARstandard)/ARstandard) * 1000
Fractionation factors[]
While an isotope effect is the physical tendency for stable isotopes to distribute in a particular way, the isotopic fractionation is the measurable result of this tendency.[5] The isotopic fractionation of a natural process can be calculated from measured isotope abundances. The calculated value is called a "fractionation factor," and allows the effect of different processes on isotope distributions to be mathematically compared.[5] For example, imagine a chemical reaction Reactant → Product. Reactant and Product are materials that both contain the element X, and X has two stable isotopes, AX (the heavy isotope, with a mass of A) and BX (the light isotope, with a mass of B). The fractionation factor for the element X in the reaction Reactant → Product is represented by the notation AαProduct/Reactant. AαProduct/Reactant is calculated as follows:[5]
AαProduct/Reactant = (δAXProduct + 1)/(δAXReactant + 1)
Fractionation factors can also be reported using the notation AεProduct/Reactant, which is sometimes called the "enrichment factor" and is calculated as follows:[5]
AεProduct/Reactant = AαProduct/Reactant - 1
Like δ values, ε values can be reported in per mille by multiplying by 1000.
Δ33S and Δ36S notation[]
All kinetic and equilibrium isotope effects result from differences in atomic mass.[3][5] As a result, a reaction that fractionates 34S will also fractionate 33S and 36S, and the fractionation factor for each isotope will be mathematically proportional to its mass.[3] Because of the mathematical relationships of their masses, the observed relationships between δ34S, δ33S, and δ36S in most natural materials are approximately δ33S = 0.515 × δ34S and δ36S = 1.90 × δ34S.[8] Rarely, natural processes can create deviations from this relationship, and these deviations are reported as Δ33S and Δ36S values, usually pronounced as "cap delta." These values are typically calculated as follows:[3][9]
Δ33S = 1000 × [(1 + δ33S/1000) - (1 + δ34S 1000)0.518 - 1]
Δ36S = 1000 × [(1 + δ36S/1000) − (1 + δ34S/1000)1.91 − 1]
However, the method for calculating Δ33S and Δ36S values is not standardized, and can differ among publications.[10]

Reference materials[]
Agreed-upon reference materials are required so that reported δ values are comparable among studies. For the sulfur isotope system, δ34S values are reported on the Vienna-Cañon Diablo Troilite (VCDT) scale.[11] The original CDT scale was based on a sample of the mineral troilite recovered from the Canyon Diablo meteorite at Meteor Crater, Arizona, US.[3] The Cañon Diablo Troilite was assigned a δ34S value of 0‰.[3] However, troilite from the Canyon Diablo meteorite was later discovered to have variable sulfur isotope composition.[12] As a result, VCDT was established as a hypothetical sulfur isotope reference with a 34R value of 0.044151[11] and δ34S of 0‰, but no physical sample of VCDT exists. Samples are now measured in comparison to International Atomic Energy Agency (IAEA) reference materials, which are well-characterized, lab-prepared compounds with known δ34S values.[13] A commonly-used IAEA reference material is IAEA-S-1, a silver sulfide reference material with a δ34S value of -0.30‰ VCDT.[4][14] 33S and 36S abundance can also be measured relative to IAEA reference materials and reported on the VCDT scale.[13] For these isotopes, too, VCDT is established as having δ33S and δ36S values of 0‰.[13] The 33R value of VCDT is 0.007877 and the 36R value is 0.0002.[13] IAEA-S-1 has a 33R value of 0.0007878 and a δ33S value of -0.05‰ VCDT; it has a δ36S value of -0.6‰ VCDT.[13]
Analytical methods and instrumentation[]
The sulfur isotopic composition of natural samples can be determined by Elemental Analysis-Isotope Ratio Mass Spectrometry (EA-IRMS),[15][16] by Dual Inlet-Isotope Ratio Mass Spectrometry (DI-IRMS),[17] by Multi-Collector-Inductively Coupled Plasma Mass Spectrometry (MC-ICPMS),[18] by Secondary Ion Mass Spectrometry (SIMS),[1][19] or by Nanoscale secondary ion mass spectrometry (NanoSIMS).[20] MC-ICPMS can be paired with gas chromatography (GC-MC-ICPMS) to separate certain volatile compounds in a sample and measure the sulfur isotopic composition of individual compounds.[21][22]
Natural variations in sulfur isotope abundance[]
Sulfur in natural materials[]
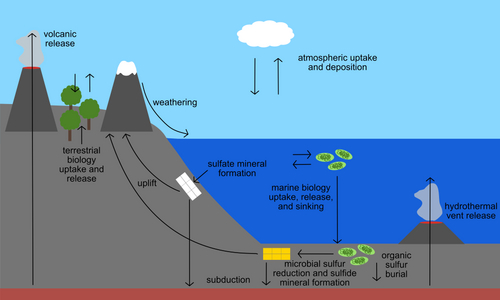
Sulfur is present in the environment in solids, gases, and aqueous species. Sulfur-containing solids on Earth include the common minerals pyrite (FeS2), galena (PbS), and gypsum (CaSO4•2H2O). Sulfur is also an important component of biological material, including in the essential amino acids cysteine and methionine, the B vitamins thiamine and biotin, and the ubiquitous substrate coenzyme A. In the ocean and other natural waters, sulfur is abundant as dissolved sulfate. Hydrogen sulfide is also present in some parts of the deep ocean where it is released from hydrothermal vents. Both sulfate and sulfide can be used by specialized microbes to obtain energy or to grow.[23] Gases including sulfur dioxide and carbonyl sulfide make up the atmospheric component of the sulfur cycle. Any process that transports or chemically transforms sulfur between these many natural materials also has the potential to fractionate sulfur isotopes.
Sulfur isotopic abundance in natural materials[]
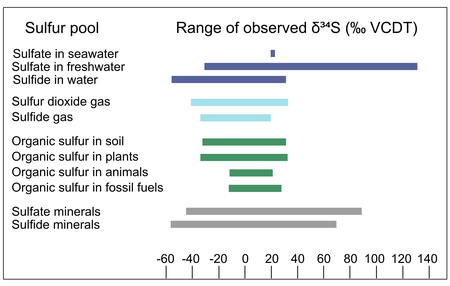
Sulfur in natural materials can vary widely in isotopic composition: compilations of the δ34S values of natural sulfur-containing materials include values ranging from -55‰ to 135‰ VCDT.[24] The ranges of δ34S values vary across sulfur-containing materials: for example, the sulfur in animal tissue ranges from ~ -10 to +20‰ VCDT, while the sulfate in natural waters ranges from ~ -20 to +135‰ VCDT.[24] The range of sulfur isotope abundances in different natural materials results from the isotope fractionation associated with natural processes like the formation and modification of those materials, discussed in the next section.
Processes that fractionate sulfur isotopes[]
Numerous natural processes are capable of fractionating sulfur isotopes. Microbes are capable of a wide variety of sulfur metabolisms, including the oxidation, reduction, and disproportionation (or simultaneous oxidation and reduction) of sulfur compounds.[1] The effect of these metabolisms on sulfur isotopic composition of the reactants and products is also highly variable, depending on the rate of relevant reactions, availability of nutrients, and other biological and environmental parameters.[25][26] As an example, the microbial reduction of sulfate to sulfide generally results in a 34S-depleted product, but the strength of this fractionation has been shown to range from 0 to 65.6‰ VCDT.[25][27]
Many abiotic processes also fractionate sulfur isotopes. Small fractionations with ε values from 0-5‰ have been observed in the formation of the mineral gypsum, an evaporite mineral produced through the evaporation of seawater.[28] Some sulfide minerals, including pyrite and galena, can form through thermochemical sulfate reduction, a process in which seawater sulfate trapped in seafloor rock is reduced to sulfide by geological heat as the rock is buried; this process generally fractionates sulfur more strongly than gypsum formation.[29]
Prior to the rise of oxygen in Earth's atmosphere (referred to as the Great Oxidation Event), additional sulfur-fractionating processes referred to as mass-anomalous or mass-independent fractionation uniquely affected the abundance of 33S and 36S in the rock record.[9] Mass-anomalous fractionations are rare, but they can occur through certain photochemical reactions of gases in the atmosphere.[30][31] Studies have shown that photochemical reactions of atmospheric sulfur dioxide can cause substantial mass-anomalous fractionation of sulfur isotopes.[30][31]
| Process | Range of observed 34ε (‰ VCDT) | Reference |
|---|---|---|
| Assimilatory sulfate reduction | -0.9 to -2.8 | [32][33][34][35] |
| Dissimilatory sulfate reduction | 0 to -65.6 | [25][27][36][37][38][39][40][41][42] |
| Sulfite reduction | +0.3 to -41 | [32][38][43] |
| Sulfide oxidation | +3 to -18.0 | [32][44][45][46][47][48] |
| Sulfur disproportionation | Sulfate: -0.6 to +20.2
Sulfide: -5.5 to -8.6 |
[49][50][51] |
| Thermochemical sulfate reduction | +10 to +25 | [29][52][53] |
| Gypsum formation | 0 to +4.2 | [28][54][55] |
Applications[]
Rise of atmospheric oxygen[]
Signatures of mass-anomalous sulfur isotope fractionation preserved in the rock record have been an important piece of evidence for understanding the Great Oxidation Event, the sudden rise of oxygen on the ancient Earth.[9][56] Nonzero values of Δ33S and Δ36S are present in the sulfur-bearing minerals of Precambrian rock formed greater than 2.45 billion years ago, but completely absent from rock less than 2.09 billion years old.[9] Multiple mechanisms have been proposed for how oxygen prevents the fingerprints of mass-anomalous fractionation from being created and preserved; nevertheless, all studies of Δ33S and Δ36S records conclude that oxygen was essentially absent from Earth's atmosphere prior to 2.45 billion years ago.[9][10][30][57][58]
Paleobiology and paleoclimate[]
A number of microbial metabolisms fractionate sulfur isotopes in distinctive ways, and the sulfur isotopic fingerprints of these metabolisms can be preserved in minerals and ancient organic matter.[1] By measuring the sulfur isotopic composition of these preserved materials, scientists can reconstruct ancient biological processes and the environments where they occurred.[1] δ34S values in the geologic record have been inferred to reveal the history of microbial sulfate reduction[59][60] and sulfide oxidation.[61] Paired δ34S and Δ33S records have also been used to show ancient microbial sulfur disproportionation.[62][26]

Microbial dissimilatory sulfate reduction (MSR), an energy-yielding metabolism performed by bacteria in anoxic environments, is associated with an especially large fractionation factor.[1] The observed 34εMSR values range from 0 to -65.6‰.[25][27][36][37][38][39][40][41] Many factors influence the size of this fractionation, including sulfate reduction rate,[32][37] sulfate concentration and transport,[25][41] availability of electron donors and other nutrients,[27][39][40] and physiological differences like protein expression.[42] Sulfide produced through MSR may then go on to form the mineral pyrite, preserving the 34S-depleted fingerprint of MSR in sedimentary rocks.[1][54] Many studies have investigated the δ34S values of ancient pyrite in order to understand past biological and environmental conditions.[1] For example, pyrite δ34S records have been used to reconstruct shifts in primary productivity levels,[63] changing ocean oxygen content,[64][65] and glacial-interglacial changes in sea level and weathering.[66] Some studies compare sulfur isotopes in pyrite to a second sulfur-containing material, like sulfate or preserved organic matter.[63][64] Comparing pyrite to another material gives a fuller picture of how sulfur moved through ancient environments: it provides clues about the size of ancient 34εMSR values and the environmental conditions controlling MSR fractionation of sulfur isotopes.[63][64]
Paleoceanography[]
δ34S records have been used to infer changes in seawater sulfate concentrations.[67] Because the δ34S values of carbonate-associated sulfate are thought to be sensitive to seawater sulfate levels, these measurements have been used to reconstruct the history of seawater sulfate.[68] δ34S values of pyrite have also been applied to reconstruct the concentration of seawater sulfate, based on expected biological fractionations at low sulfate concentrations.[69][70] Both of these methods rely on assumptions about the depositional environment or the biological community, creating some uncertainty in the resulting reconstructions.[25][68]
See also[]
- Δ34S
- Isotopes of sulfur
- Hydrogen isotope biogeochemistry
- Stable isotope ratio
- Isotope analysis
- Isotope geochemistry
References[]
- ^ a b c d e f g h Canfield, D. E. (2001-01-01). "Biogeochemistry of Sulfur Isotopes". Reviews in Mineralogy and Geochemistry. 43 (1): 607–636. Bibcode:2001RvMG...43..607C. doi:10.2138/gsrmg.43.1.607. ISSN 1529-6466.
- ^ a b Wang, Meng; Audi, G.; Kondev, F. G.; Huang, W.J.; Naimi, S.; Xu, Xing (2017). "The AME2016 atomic mass evaluation (II). Tables, graphs and references". Chinese Physics C. 41 (3): 030003. Bibcode:2017ChPhC..41c0003W. doi:10.1088/1674-1137/41/3/030003. hdl:11858/00-001M-0000-0010-23E8-5. ISSN 1674-1137.
- ^ a b c d e f g h i j k l m Sharp, Zachary (2006). Principles of Stable Isotope Geochemistry.
- ^ a b Brand, Willi; Coplen, Tyler; Vogel, Jochen; Rosner, Martin; Prohaska, Thomas (2014). "Assessment of international reference materials for isotope-ratio analysis (IUPAC Technical Report)". Pure and Applied Chemistry. 86 (3): 425–467. doi:10.1515/pac-2013-1023. hdl:11858/00-001M-0000-0023-C6D8-8. S2CID 98812517.
- ^ a b c d e f g h i j k l m n o p Hayes, John (2002). Practices and Principles of Stable Isotopic Measurement in Organic Geochemistry (Revision 2).
- ^ Mccready, R. G. L.; Laishley, E. J.; Krouse, H. R. (1976-08-01). "Biogeochemical implications of inverse sulfur isotope effects during reduction of sulfur compounds by Clostridium pasteurianum". Geochimica et Cosmochimica Acta. 40 (8): 979–981. Bibcode:1976GeCoA..40..979M. doi:10.1016/0016-7037(76)90146-0. ISSN 0016-7037.
- ^ Craig, H. and Gordon, L. I. 1965. “Deuterium and oxygen 18 variations in the ocean and the marine atmosphere”. In Stable Isotopes in Oceanographic Studies and Paleotemperatures, Edited by: Tongiorgi, E. 9–130. Pisa: Laboratorio di Geologia Nucleare.
- ^ Hulston, J. R.; Thode, H. G. (1965). "Variations in the S33, S34, and S36 contents of meteorites and their relation to chemical and nuclear effects". Journal of Geophysical Research. 70 (14): 3475–3484. Bibcode:1965JGR....70.3475H. doi:10.1029/JZ070i014p03475. ISSN 2156-2202.
- ^ a b c d e Farquhar, James; Bao, Huiming; Thiemens, Mark (2000-08-04). "Atmospheric Influence of Earth's Earliest Sulfur Cycle". Science. 289 (5480): 756–758. Bibcode:2000Sci...289..756F. doi:10.1126/science.289.5480.756. ISSN 0036-8075. PMID 10926533.
- ^ a b Halevy, Itay; Johnston, David T.; Schrag, Daniel P. (2010-07-09). "Explaining the Structure of the Archean Mass-Independent Sulfur Isotope Record". Science. 329 (5988): 204–207. Bibcode:2010Sci...329..204H. doi:10.1126/science.1190298. ISSN 0036-8075. PMID 20508089. S2CID 45825809.
- ^ a b Ding, Tiping; Bai, Ruimei; Li, Yanhe; Wan, Defang; Zou, Xiaoqiu; Zhang, Qinglian (1999-08-01). "Determination of the absolute32S/34S ratio of IAEA-S-1 reference material and V-CDT sulfur isotope standard". Science in China Series D: Earth Sciences. 42 (1): 45–51. Bibcode:1999ScChD..42...45D. doi:10.1007/BF02878497. ISSN 1862-2801. S2CID 93625037.
- ^ Beaudoin, Georges; Taylor, B. E.; Rumble, D.; Thiemens, M. (1994-10-01). "Variations in the sulfur isotope composition of troilite from the Cañon Diablo iron meteorite". Geochimica et Cosmochimica Acta. 58 (19): 4253–4255. Bibcode:1994GeCoA..58.4253B. doi:10.1016/0016-7037(94)90277-1. ISSN 0016-7037.
- ^ a b c d e Ding, T.; Valkiers, S.; Kipphardt, H.; De Bièvre, P.; Taylor, P. D. P.; Gonfiantini, R.; Krouse, R. (2001-08-01). "Calibrated sulfur isotope abundance ratios of three IAEA sulfur isotope reference materials and V-CDT with a reassessment of the atomic weight of sulfur". Geochimica et Cosmochimica Acta. 65 (15): 2433–2437. Bibcode:2001GeCoA..65.2433D. doi:10.1016/S0016-7037(01)00611-1. ISSN 0016-7037.
- ^ Krouse, H. R.; Coplen, Tyler B. (1997-02-28). "Reporting of relative sulfur isotope-ratio data (Technical Report)". Pure and Applied Chemistry. 69 (2): 293–296. doi:10.1351/pac199769020293. ISSN 0033-4545. S2CID 95627976.
- ^ Giesemann, A.; Jaeger, H.-J.; Norman, A. L.; Krouse, H. R.; Brand, W. A. (1994-09-15). "Online Sulfur-Isotope Determination Using an Elemental Analyzer Coupled to a Mass Spectrometer". Analytical Chemistry. 66 (18): 2816–2819. doi:10.1021/ac00090a005. ISSN 0003-2700.
- ^ Grassineau, Nathalie V. (2006-05-01). "High-precision EA-IRMS analysis of S and C isotopes in geological materials". Applied Geochemistry. Frontiers in Analytical Geochemistry–An IGC 2004 Perspective. 21 (5): 756–765. Bibcode:2006ApGC...21..756G. doi:10.1016/j.apgeochem.2006.02.015. ISSN 0883-2927.
- ^ Mayer, Bernhard; Krouse, H. Roy (2004-01-01), de Groot, Pier A. (ed.), "Chapter 26 - Procedures for Sulfur Isotope Abundance Studies", Handbook of Stable Isotope Analytical Techniques, Elsevier, pp. 538–596, ISBN 978-0-444-51114-0, retrieved 2020-05-25
- ^ Paris, Guillaume; Sessions, Alex L.; Subhas, Adam V.; Adkins, Jess F. (2013-05-08). "MC-ICP-MS measurement of δ34S and ∆33S in small amounts of dissolved sulfate". Chemical Geology. 345: 50–61. Bibcode:2013ChGeo.345...50P. doi:10.1016/j.chemgeo.2013.02.022. ISSN 0009-2541.
- ^ Riciputi, Lee R; Paterson, Bruce A; Ripperdan, Robert L (1998-10-19). "Measurement of light stable isotope ratios by SIMS:: Matrix effects for oxygen, carbon, and sulfur isotopes in minerals33Dedicated to the memory of Al Nier". International Journal of Mass Spectrometry. 178 (1): 81–112. doi:10.1016/S1387-3806(98)14088-5. ISSN 1387-3806.
- ^ Hauri, Erik H.; Papineau, Dominic; Wang, Jianhua; Hillion, Francois (2016-01-20). "High-precision analysis of multiple sulfur isotopes using NanoSIMS" (PDF). Chemical Geology. 420: 148–161. Bibcode:2016ChGeo.420..148H. doi:10.1016/j.chemgeo.2015.11.013. ISSN 0009-2541.
- ^ Amrani, Alon; Sessions, Alex L.; Adkins, Jess F. (2009-11-01). "Compound-Specific δ34S Analysis of Volatile Organics by Coupled GC/Multicollector-ICPMS". Analytical Chemistry. 81 (21): 9027–9034. doi:10.1021/ac9016538. ISSN 0003-2700. PMID 19807109.
- ^ Said‐Ahmad, Ward; Amrani, Alon (2013). "A sensitive method for the sulfur isotope analysis of dimethyl sulfide and dimethylsulfoniopropionate in seawater". Rapid Communications in Mass Spectrometry. 27 (24): 2789–2796. Bibcode:2013RCMS...27.2789S. doi:10.1002/rcm.6751. ISSN 1097-0231. PMID 24214865.
- ^ Canfield, Donald E.; Erik Kristensen; Bo Thamdrup (2005-01-01), Canfield, Donald E.; Kristensen, Erik; Thamdrup, Bo (eds.), "The Sulfur Cycle", Advances in Marine Biology, Aquatic Geomicrobiology, Academic Press, vol. 48, pp. 313–381, doi:10.1016/S0065-2881(05)48009-8, ISBN 9780120261475, retrieved 2020-05-23
- ^ a b Meija, Juris; Coplen, Tyler; Berglund, Michael; Brand, Willi; De Bievre, Paul; Groening, Manfred; Holden, Norman; Irrgeher, Johanna; Loss, Robert; Walczyk, Thomas; Prohaska, Thomas (2013). "Atomic weights of the elements 2013 (IUPAC Technical Report)". Pure and Applied Chemistry. 88.
- ^ a b c d e f Bradley, A. S.; Leavitt, W. D.; Schmidt, M.; Knoll, A. H.; Girguis, P. R.; Johnston, D. T. (January 2016). "Patterns of sulfur isotope fractionation during microbial sulfate reduction". Geobiology. 14 (1): 91–101. doi:10.1111/gbi.12149. PMID 26189479.
- ^ a b Johnston, David T.; Farquhar, James; Wing, Boswell A.; Kaufman, Alan J.; Canfield, Donald E.; Habicht, Kirsten S. (2005-06-01). "Multiple sulfur isotope fractionations in biological systems: A case study with sulfate reducers and sulfur disproportionators". American Journal of Science. 305 (6–8): 645–660. Bibcode:2005AmJS..305..645J. doi:10.2475/ajs.305.6-8.645. ISSN 0002-9599.
- ^ a b c d Sim, Min Sub; Bosak, Tanja; Ono, Shuhei (2011-07-01). "Large Sulfur Isotope Fractionation Does Not Require Disproportionation". Science. 333 (6038): 74–77. Bibcode:2011Sci...333...74S. doi:10.1126/science.1205103. ISSN 0036-8075. PMID 21719675. S2CID 1248182.
- ^ a b Raab, M.; Spiro, B. (1991-04-05). "Sulfur isotopic variations during seawater evaporation with fractional crystallization". Chemical Geology: Isotope Geoscience Section. 86 (4): 323–333. doi:10.1016/0168-9622(91)90014-N. ISSN 0168-9622.
- ^ a b Machel, Hans G.; Krouse, H. Roy; Sassen, Roger (1995-07-01). "Products and distinguishing criteria of bacterial and thermochemical sulfate reduction". Applied Geochemistry. 10 (4): 373–389. Bibcode:1995ApGC...10..373M. doi:10.1016/0883-2927(95)00008-8. ISSN 0883-2927.
- ^ a b c Masterson, Andrew L.; Farquhar, James; Wing, Boswell A. (2011-06-15). "Sulfur mass-independent fractionation patterns in the broadband UV photolysis of sulfur dioxide: Pressure and third body effects". Earth and Planetary Science Letters. 306 (3): 253–260. Bibcode:2011E&PSL.306..253M. doi:10.1016/j.epsl.2011.04.004. ISSN 0012-821X.
- ^ a b Lyons, James R. (2007). "Mass-independent fractionation of sulfur isotopes by isotope-selective photodissociation of SO2". Geophysical Research Letters. 34 (22): L22811. Bibcode:2007GeoRL..3422811L. doi:10.1029/2007GL031031. ISSN 1944-8007.
- ^ a b c d Kaplan, I. R.; Rittenberg, S. C. (1964). "Microbiological Fractionation of Sulphur Isotopes". Microbiology. 34 (2): 195–212. doi:10.1099/00221287-34-2-195. ISSN 1350-0872. PMID 14135528.
- ^ Trust, B. A.; Fry, B. (1992). "Stable sulphur isotopes in plants: a review". Plant, Cell & Environment. 15 (9): 1105–1110. doi:10.1111/j.1365-3040.1992.tb01661.x. ISSN 1365-3040.
- ^ Patron, Nicola J.; Durnford, Dion G.; Kopriva, Stanislav (2008-02-04). "Sulfate assimilation in eukaryotes: fusions, relocations and lateral transfers". BMC Evolutionary Biology. 8 (1): 39. doi:10.1186/1471-2148-8-39. ISSN 1471-2148. PMC 2275785. PMID 18248682.
- ^ Schiff, J. A.; Fankhauser, H. (1981). Bothe, Hermann; Trebst, Achim (eds.). "Assimilatory Sulfate Reduction". Biology of Inorganic Nitrogen and Sulfur. Proceedings in Life Sciences. Berlin, Heidelberg: Springer: 153–168. doi:10.1007/978-3-642-67919-3_11. ISBN 978-3-642-67919-3.
- ^ a b Detmers, Jan; Brüchert, Volker; Habicht, Kirsten S.; Kuever, Jan (2001-02-01). "Diversity of Sulfur Isotope Fractionations by Sulfate-Reducing Prokaryotes". Applied and Environmental Microbiology. 67 (2): 888–894. Bibcode:2001ApEnM..67..888D. doi:10.1128/AEM.67.2.888-894.2001. ISSN 0099-2240. PMC 92663. PMID 11157259.
- ^ a b c Canfield, D. E. (2001-04-01). "Isotope fractionation by natural populations of sulfate-reducing bacteria". Geochimica et Cosmochimica Acta. 65 (7): 1117–1124. Bibcode:2001GeCoA..65.1117C. doi:10.1016/S0016-7037(00)00584-6. ISSN 0016-7037.
- ^ a b c Kemp, A. L. W.; Thode, H. G. (1968-01-01). "The mechanism of the bacterial reduction of sulphate and of sulphite from isotope fractionation studies". Geochimica et Cosmochimica Acta. 32 (1): 71–91. Bibcode:1968GeCoA..32...71K. doi:10.1016/0016-7037(68)90088-4. ISSN 0016-7037.
- ^ a b c Sim, Min Sub; Ono, Shuhei; Donovan, Katie; Templer, Stefanie P.; Bosak, Tanja (2011-08-01). "Effect of electron donors on the fractionation of sulfur isotopes by a marine Desulfovibrio sp". Geochimica et Cosmochimica Acta. 75 (15): 4244–4259. Bibcode:2011GeCoA..75.4244S. doi:10.1016/j.gca.2011.05.021. ISSN 0016-7037.
- ^ a b c Sim, Min Sub; Ono, Shuhei; Bosak, Tanja (2012-12-01). "Effects of Iron and Nitrogen Limitation on Sulfur Isotope Fractionation during Microbial Sulfate Reduction". Applied and Environmental Microbiology. 78 (23): 8368–8376. Bibcode:2012ApEnM..78.8368S. doi:10.1128/AEM.01842-12. ISSN 0099-2240. PMC 3497358. PMID 23001667.
- ^ a b c Sim, Min Sub; Paris, Guillaume; Adkins, Jess F.; Orphan, Victoria J.; Sessions, Alex L. (2017-06-01). "Quantification and isotopic analysis of intracellular sulfur metabolites in the dissimilatory sulfate reduction pathway". Geochimica et Cosmochimica Acta. 206: 57–72. Bibcode:2017GeCoA.206...57S. doi:10.1016/j.gca.2017.02.024. ISSN 0016-7037.
- ^ a b Leavitt, William D.; Venceslau, Sofia S.; Pereira, Inês A. C.; Johnston, David T.; Bradley, Alexander S. (2016-10-01). "Fractionation of sulfur and hydrogen isotopes in Desulfovibrio vulgaris with perturbed DsrC expression". FEMS Microbiology Letters. 363 (20): fnw226. doi:10.1093/femsle/fnw226. ISSN 0378-1097. PMID 27702753.
- ^ Leavitt, William D.; Cummins, Renata; Schmidt, Marian L.; Sim, Min S.; Ono, Shuhei; Bradley, Alexander S.; Johnston, David T. (2014). "Multiple sulfur isotope signatures of sulfite and thiosulfate reduction by the model dissimilatory sulfate-reducer, Desulfovibrio alaskensis str. G20". Frontiers in Microbiology. 5: 591. doi:10.3389/fmicb.2014.00591. ISSN 1664-302X. PMC 4243691. PMID 25505449.
- ^ Balci, Nurgul; Shanks, Wayne C.; Mayer, Bernhard; Mandernack, Kevin W. (2007-08-01). "Oxygen and sulfur isotope systematics of sulfate produced by bacterial and abiotic oxidation of pyrite". Geochimica et Cosmochimica Acta. 71 (15): 3796–3811. Bibcode:2007GeCoA..71.3796B. doi:10.1016/j.gca.2007.04.017. ISSN 0016-7037.
- ^ Howard Gest, Brian Fry; Hayes, J. M. (1984-05-01). "Isotope effects associated with the anaerobic oxidation of sulfide by the purple photosynthetic bacterium, Chromatium vinosum". FEMS Microbiology Letters. 22 (3): 283–287. doi:10.1111/j.1574-6968.1984.tb00742.x. ISSN 0378-1097.
- ^ Jones, Galen E.; Starkey, Robert L. (March 1957). "Fractionation of Stable Isotopes of Sulfur by Microorganisms and Their Role in Deposition of Native Sulfur". Applied Microbiology. 5 (2): 111–118. doi:10.1128/AEM.5.2.111-118.1957. ISSN 0003-6919. PMC 1057268. PMID 13425528.
- ^ Kaplan, I. R.; Rafter, T. A. (1958-03-07). "Fractionation of Stable Isotopes of Sulfur by Thiobacilli". Science. 127 (3297): 517–518. Bibcode:1958Sci...127..517K. doi:10.1126/science.127.3297.517. ISSN 0036-8075. PMID 13529013.
- ^ Nakai, Nobuyuki; Jensen, M. L. (1964-12-01). "The kinetic isotope effect in the bacterial reduction and oxidation of sulfur". Geochimica et Cosmochimica Acta. 28 (12): 1893–1912. Bibcode:1964GeCoA..28.1893N. doi:10.1016/0016-7037(64)90136-X. ISSN 0016-7037.
- ^ Canfield, D. E.; Thamdrup, B. (1994-12-23). "The production of 34S-depleted sulfide during bacterial disproportionation of elemental sulfur". Science. 266 (5193): 1973–1975. Bibcode:1994Sci...266.1973C. doi:10.1126/science.11540246. ISSN 0036-8075. PMID 11540246.
- ^ Canfield, D. E.; Thamdrup, B.; Fleischer, S. (1998). "Isotope fractionation and sulfur metabolism by pure and enrichment cultures of elemental sulfur-disproportionating bacteria". Limnology and Oceanography. 43 (2): 253–264. Bibcode:1998LimOc..43..253C. doi:10.4319/lo.1998.43.2.0253. ISSN 1939-5590.
- ^ Böttcher, Michael E; Thamdrup, Bo (2001-05-15). "Anaerobic sulfide oxidation and stable isotope fractionation associated with bacterial sulfur disproportionation in the presence of MnO2". Geochimica et Cosmochimica Acta. 65 (10): 1573–1581. Bibcode:2001GeCoA..65.1573B. doi:10.1016/S0016-7037(00)00622-0. ISSN 0016-7037.
- ^ Amrani, Alon; Deev, Andrei; Sessions, Alex L.; Tang, Yongchun; Adkins, Jess F.; Hill, Ronald J.; Moldowan, J. Michael; Wei, Zhibin (2012-05-01). "The sulfur-isotopic compositions of benzothiophenes and dibenzothiophenes as a proxy for thermochemical sulfate reduction". Geochimica et Cosmochimica Acta. 84: 152–164. Bibcode:2012GeCoA..84..152A. doi:10.1016/j.gca.2012.01.023. ISSN 0016-7037.
- ^ Kiyosu, Yasuhiro; Krouse, H. Roy (1993). "Thermochemical reduction and sulfur isotopic behavior of sulfate by acetic acid in the presence of native sulfur". Geochemical Journal. 27 (1): 49–57. Bibcode:1993GeocJ..27...49K. doi:10.2343/geochemj.27.49.
- ^ a b Ault, W. U; Kulp, J. L (1959-07-01). "Isotopic geochemistry of sulphur". Geochimica et Cosmochimica Acta. 16 (4): 201–235. Bibcode:1959GeCoA..16..201A. doi:10.1016/0016-7037(59)90112-7. ISSN 0016-7037.
- ^ Thode, H. G; Monster, J; Dunford, H. B (1961-11-01). "Sulphur isotope geochemistry". Geochimica et Cosmochimica Acta. 25 (3): 159–174. Bibcode:1961GeCoA..25..159T. doi:10.1016/0016-7037(61)90074-6. ISSN 0016-7037.
- ^ Sessions, Alex L.; Doughty, David M.; Welander, Paula V.; Summons, Roger E.; Newman, Dianne K. (2009-07-28). "The Continuing Puzzle of the Great Oxidation Event". Current Biology. 19 (14): R567–R574. doi:10.1016/j.cub.2009.05.054. hdl:1721.1/96187. ISSN 0960-9822. PMID 19640495. S2CID 7346329.
- ^ Pavlov, A.a.; Kasting, J.f. (2002-03-01). "Mass-Independent Fractionation of Sulfur Isotopes in Archean Sediments: Strong Evidence for an Anoxic Archean Atmosphere". Astrobiology. 2 (1): 27–41. Bibcode:2002AsBio...2...27P. doi:10.1089/153110702753621321. ISSN 1531-1074. PMID 12449853.
- ^ Zahnle, K.; Claire, M.; Catling, D. (2006). "The loss of mass-independent fractionation in sulfur due to a Palaeoproterozoic collapse of atmospheric methane". Geobiology. 4 (4): 271–283. doi:10.1111/j.1472-4669.2006.00085.x. ISSN 1472-4669.
- ^ Shen, Yanan; Farquhar, James; Masterson, Andrew; Kaufman, Alan J.; Buick, Roger (2009-03-30). "Evaluating the role of microbial sulfate reduction in the early Archean using quadruple isotope systematics". Earth and Planetary Science Letters. 279 (3): 383–391. Bibcode:2009E&PSL.279..383S. doi:10.1016/j.epsl.2009.01.018. ISSN 0012-821X.
- ^ Shen, Yanan; Buick, Roger (2004-02-01). "The antiquity of microbial sulfate reduction". Earth-Science Reviews. 64 (3): 243–272. Bibcode:2004ESRv...64..243S. doi:10.1016/S0012-8252(03)00054-0. ISSN 0012-8252.
- ^ Canfield, Donald E.; Teske, Andreas (July 1996). "Late Proterozoic rise in atmospheric oxygen concentration inferred from phylogenetic and sulphur-isotope studies". Nature. 382 (6587): 127–132. Bibcode:1996Natur.382..127C. doi:10.1038/382127a0. ISSN 1476-4687. PMID 11536736. S2CID 4360682.
- ^ Bontognali, Tomaso R. R.; Sessions, Alex L.; Allwood, Abigail C.; Fischer, Woodward W.; Grotzinger, John P.; Summons, Roger E.; Eiler, John M. (2012-09-18). "Sulfur isotopes of organic matter preserved in 3.45-billion-year-old stromatolites reveal microbial metabolism". Proceedings of the National Academy of Sciences. 109 (38): 15146–15151. doi:10.1073/pnas.1207491109. ISSN 0027-8424. PMC 3458326. PMID 22949693.
- ^ a b c Fike, D. A.; Grotzinger, J. P. (2008-06-01). "A paired sulfate–pyrite δ34S approach to understanding the evolution of the Ediacaran–Cambrian sulfur cycle". Geochimica et Cosmochimica Acta. 72 (11): 2636–2648. Bibcode:2008GeCoA..72.2636F. doi:10.1016/j.gca.2008.03.021. ISSN 0016-7037.
- ^ a b c Raven, Morgan Reed; Fike, David A.; Bradley, Alexander S.; Gomes, Maya L.; Owens, Jeremy D.; Webb, Samuel A. (2019-04-15). "Paired organic matter and pyrite δ34S records reveal mechanisms of carbon, sulfur, and iron cycle disruption during Ocean Anoxic Event 2". Earth and Planetary Science Letters. 512: 27–38. Bibcode:2019E&PSL.512...27R. doi:10.1016/j.epsl.2019.01.048. ISSN 0012-821X.
- ^ Owens, Jeremy D.; Gill, Benjamin C.; Jenkyns, Hugh C.; Bates, Steven M.; Severmann, Silke; Kuypers, Marcel M. M.; Woodfine, Richard G.; Lyons, Timothy W. (2013-11-12). "Sulfur isotopes track the global extent and dynamics of euxinia during Cretaceous Oceanic Anoxic Event 2". Proceedings of the National Academy of Sciences. 110 (46): 18407–18412. Bibcode:2013PNAS..11018407O. doi:10.1073/pnas.1305304110. ISSN 0027-8424. PMC 3831968. PMID 24170863.
- ^ Pasquier, Virgil; Sansjofre, Pierre; Rabineau, Marina; Revillon, Sidonie; Houghton, Jennifer; Fike, David A. (2017-06-06). "Pyrite sulfur isotopes reveal glacial−interglacial environmental changes". Proceedings of the National Academy of Sciences. 114 (23): 5941–5945. Bibcode:2017PNAS..114.5941P. doi:10.1073/pnas.1618245114. ISSN 0027-8424. PMC 5468663. PMID 28533378.
- ^ Fike, David A.; Bradley, Alexander S.; Rose, Catherine V. (2015-05-30). "Rethinking the Ancient Sulfur Cycle". Annual Review of Earth and Planetary Sciences. 43 (1): 593–622. Bibcode:2015AREPS..43..593F. doi:10.1146/annurev-earth-060313-054802. ISSN 0084-6597.
- ^ a b Kah, Linda C.; Lyons, Timothy W.; Frank, Tracy D. (October 2004). "Low marine sulphate and protracted oxygenation of the Proterozoic biosphere". Nature. 431 (7010): 834–838. Bibcode:2004Natur.431..834K. doi:10.1038/nature02974. ISSN 1476-4687. PMID 15483609. S2CID 4404486.
- ^ Habicht, Kirsten S.; Gade, Michael; Thamdrup, Bo; Berg, Peter; Canfield, Donald E. (2002-12-20). "Calibration of Sulfate Levels in the Archean Ocean". Science. 298 (5602): 2372–2374. Bibcode:2002Sci...298.2372H. doi:10.1126/science.1078265. ISSN 0036-8075. PMID 12493910. S2CID 188175.
- ^ Crowe, Sean A.; Paris, Guillaume; Katsev, Sergei; Jones, CarriAyne; Kim, Sang-Tae; Zerkle, Aubrey L.; Nomosatryo, Sulung; Fowle, David A.; Adkins, Jess F.; Sessions, Alex L.; Farquhar, James (2014-11-07). "Sulfate was a trace constituent of Archean seawater". Science. 346 (6210): 735–739. Bibcode:2014Sci...346..735C. doi:10.1126/science.1258966. ISSN 0036-8075. PMID 25378621. S2CID 206561027.
- Sulfur
- Isotopes of sulfur
- Biogeochemistry