Synapsid
| Synapsids Temporal range: Pennsylvanian–Holocene, 312–0 Ma
PreꞒ
Ꞓ
O
S
D
C
P
T
J
K
Pg
N
| |
|---|---|

| |
| Examples of synapsids: Cotylorhynchus, Oedaleops (two caseasaurs), Edaphosaurus, Dimetrodon, Inostrancevia, Placerias, platypus and humans (four therapsids including two mammals). | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Superclass: | Tetrapoda |
| Clade: | Reptiliomorpha |
| Clade: | Amniota |
| Clade: | Synapsida Osborn, 1903 |
| Subgroups | |
| |
| Synonyms | |
|
Theropsida Seeley, 1895[3] | |
Synapsids[a] are a group of animals that includes mammals and every animal more closely related to mammals than to the other members of the amniote clade, such as reptiles and birds.[4] Unlike other amniotes, they have a temporal fenestra, an opening low in the skull roof behind each eye, leaving a bony arch beneath each; this accounts for their name.[5] Primitive synapsids are usually called pelycosaurs or pelycosaur-grade synapsids. This informal term consists of all synapsids that are not therapsids, a monophyletic, more advanced, mammal-like group. The non-mammalian synapsids were described as mammal-like reptiles in classical systematics, but this misleading terminology is no longer in use[6][7] as synapsids as a whole are no longer considered reptiles.[8] They are now more correctly referred to as stem mammals or proto-mammals.[9]
Synapsids evolved from basal amniotes and are one of the two major groups of amniotes, the other being the sauropsids, the group that includes reptiles and birds. The distinctive temporal fenestra developed in the ancestral synapsid about 312 million years ago, during the Late Carboniferous period.
Synapsids were the largest terrestrial vertebrates in the Permian period, 299 to 251 million years ago, equaled only by some large pareiasaurs at the end of the Permian. Their numbers and variety were severely reduced by the Permian–Triassic extinction. By the time of the extinction at the end of the Permian, all the older forms of synapsids (known as pelycosaurs) were gone, having been replaced by the more advanced therapsids. Although the dicynodonts and eutheriodonts, the latter consisting of the Eutherocephalia (Therocephalia) and Epicynodontia (Cynodontia), continued into the Triassic period as the only known surviving therapsids, archosaurs became the largest and most numerous land vertebrates during this period. There were still some large forms: Lisowicia bojani, a large dicynodont from the Late Triassic, was the size of an elephant. The cynodont group Probainognathia, which includes Mammaliaformes, were the only synapsids to survive beyond the Triassic.[10] After the Cretaceous–Paleogene extinction event, the mammalian synapsids diversified again to become the largest land and marine animals on Earth.
Linnaean and cladistic classifications[]
Synapsids as a reptilian subclass[]
At the turn of the 20th century synapsids were thought to be one of the four main subclasses of reptiles. They were differentiated from other reptiles by their distinctive temporal openings. These openings in the skull bones allowed the attachment of larger jaw muscles, hence a more efficient bite. Synapsids were considered to be the reptilian lineage that became mammals by gradually evolving increasingly mammalian features, hence the name "mammal-like reptiles", which became the broad, traditional description for all Paleozoic synapsids.[6][7]
The "mammal-like reptiles"[]
The term "mammal-like reptiles" is still used colloquially, but it is used with increasing rarity in technical literature, as it reflects a superseded understanding of these animals' evolutionary relationships. Phylogenetically, it is now understood that synapsids comprise an independent branch of the tree of life.[11] This terminology reflects the modern cladistical approach to animal relationships, according to which the only valid groups are those that include all of the descendants of a common ancestor: these are known as monophyletic groups, or clades. The term "mammal-like reptiles" includes groups that are not united in this way, which makes it a paraphyletic term. The monophyly of Synapsida is not in doubt, however, and the expressions such as "Synapsida contains the mammals" and "synapsids gave rise to the mammals" both express the same phylogenetic hypothesis.
Additionally, Reptilia has been revised into a monophyletic group and is considered entirely distinct from Synapsida, being the sister group of Synapsida within Amniota.[12]
Although Synapsida includes modern mammals, the term is most often used when referring to non-mammalian, non-therapsid synapsids.
Primitive and advanced synapsids[]
The synapsids are traditionally divided into a primitive group and an advanced group, known respectively as pelycosaurs and therapsids. 'Pelycosaurs' make up the six most primitive families of synapsids.[13] They were all rather lizard-like, with sprawling gait and possibly horny scutes. The therapsids contain the more advanced synapsids, having a more erect pose and possibly hair, at least in some forms. In traditional taxonomy, the Synapsida encompasses two distinct grades successively closer to mammals: the low-slung pelycosaurs have given rise to the more erect therapsids, who in their turn have given rise to the mammals. In traditional vertebrate classification, the Pelycosauria and Therapsida were both considered orders of the subclass Synapsida.[5][6]
In phylogenetic nomenclature, the terms are used somewhat differently, as the daughter clades are included. Most papers published during the 21st century have treated "Pelycosauria" as an informal grouping of primitive members. Therapsida has remained in use as a clade containing both the traditional therapsid families and mammals. However, in practical usage, the terms are used almost exclusively when referring to the more basal members that lie outside of Mammaliaformes.
Characteristics[]
Temporal openings[]
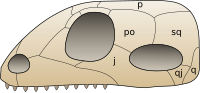
Synapsids evolved a temporal fenestra behind each eye orbit on the lateral surface of the skull. It may have provided new attachment sites for jaw muscles. A similar development took place in the diapsids, which evolved two rather than one opening behind each eye. Originally, the openings in the skull left the inner cranium covered only by the jaw muscles, but in higher therapsids and mammals, the sphenoid bone has expanded to close the opening. This has left the lower margin of the opening as an arch extending from the lower edges of the braincase.
Teeth[]

Synapsids are characterized by having differentiated teeth. These include the canines, molars, and incisors.[14] The trend towards differentiation is found in some labyrinthodonts and early anapsid reptilians in the form of enlargement of the first teeth on the maxilla, forming a form of protocanines. This trait was subsequently lost in the sauropsid line, but developed further in the synapsids. Early synapsids could have two or even three enlarged "canines", but in the therapsids, the pattern had settled to one canine in each upper jaw half. The lower canines developed later.
Jaw[]
The jaw transition is a good classification tool, as most other fossilized features that make a chronological progression from a reptile-like to a mammalian condition follow the progression of the jaw transition. The mandible, or lower jaw, consists of a single, tooth-bearing bone in mammals (the dentary), whereas the lower jaw of modern and prehistoric reptiles consists of a conglomeration of smaller bones (including the dentary, articular, and others). As they evolved in synapsids, these jaw bones were reduced in size and either lost or, in the case of the articular, gradually moved into the ear, forming one of the middle ear bones: while modern mammals possess the malleus, incus and stapes, basal synapsids (like all other tetrapods) possess only a stapes. The malleus is derived from the articular (a lower jaw bone), while the incus is derived from the quadrate (a cranial bone).[15]
Mammalian jaw structures are also set apart by the dentary-squamosal jaw joint. In this form of jaw joint, the dentary forms a connection with a depression in the squamosal known as the glenoid cavity. In contrast, all other jawed vertebrates, including reptiles and nonmammalian synapsids, possess a jaw joint in which one of the smaller bones of the lower jaw, the articular, makes a connection with a bone of the cranium called the quadrate bone to form the articular-quadrate jaw joint. In forms transitional to mammals, the jaw joint is composed of a large, lower jaw bone (similar to the dentary found in mammals) that does not connect to the squamosal, but connects to the quadrate with a receding articular bone.
Palate[]
Over time, as synapsids became more mammalian and less 'reptilian', they began to develop a secondary palate, separating the mouth and nasal cavity. In early synapsids, a secondary palate began to form on the sides of the maxilla, still leaving the mouth and nostril connected.
Eventually, the two sides of the palate began to curve together, forming a U shape instead of a C shape. The palate also began to extend back toward the throat, securing the entire mouth and creating a full palatine bone. The maxilla is also closed completely. In fossils of one of the first eutheriodonts, the beginnings of a palate are clearly visible. The later Thrinaxodon has a full and completely closed palate, forming a clear progression.[16]
Skin and fur[]

In addition to the glandular skin covered in fur found in most modern mammals, modern and extinct synapsids possess a variety of modified skin coverings, including osteoderms (bony armor embedded in the skin), scutes (protective structures of the dermis often with a horny covering), hair or fur, and scale-like structures (often formed from modified hair, as in pangolins and some rodents). While the skin of reptiles is rather thin, that of mammals has a thick dermal layer.[17]
The ancestral skin type of synapsids has been subject to discussion. Among the early synapsids, only two species of small varanopids have been found to possess scutes;[18] fossilized rows of osteoderms indicate horny armour on the neck and back, and skin impressions indicate some possessed rectangular scutes on their undersides and tails.[19][20] The pelycosaur scutes probably were nonoverlapping dermal structures with a horny overlay, like those found in modern crocodiles and turtles. These differed in structure from the scales of lizards and snakes, which are an epidermal feature (like mammalian hair or avian feathers).[21] Recently, skin impressions from the genus Ascendonanus suggest that at least varanopsids developed scales similar to those of squamates.[22]
It is currently unknown exactly when mammalian characteristics such as body hair and mammary glands first appeared, as the fossils only rarely provide direct evidence for soft tissues. An exceptionally well-preserved skull of Estemmenosuchus, a therapsid from the Upper Permian, preserves smooth skin with what appear to be glandular depressions,[23] an animal noted as being semi-aquatic.[24] The oldest known fossil showing unambiguous imprints of hair is the Callovian (late middle Jurassic) Castorocauda and several contemporary haramiyidans, both non-mammalian mammaliaform[25][26] (see below, however). More primitive members of the Cynodontia are also hypothesized to have had fur or a fur-like covering based on their inferred warm-blooded metabolism.[27] While more direct evidence of fur in early cynodonts has been proposed in the form of small pits on the snout possibly associated with whiskers, such pits are also found in some reptiles that lack whiskers.[27] There is evidence that some other non-mammalian cynodonts more basal than Castorocauda, such as Morganucodon, had Harderian glands, which are associated with the grooming and maintenance of fur. The apparent absence of these glands in non-mammaliaformes may suggest that fur did not originate until that point in synapsid evolution.[27] It is possible that fur and associated features of true warm-bloodedness did not appear until some synapsids became extremely small and nocturnal, necessitating a higher metabolism.[27]
However, Permian coprolites from Russia showcase that at least some synapsids did already have fur in this epoch. These are the oldest impressions of hair on synapsids.[28]
Mammary glands[]
Early synapsids, as far back as their known evolutionary debut in the Late Carboniferous period,[29] may have laid parchment-shelled (leathery) eggs,[30] which lacked a calcified layer, as most modern reptiles and monotremes do. This may also explain why there is no fossil evidence for synapsid eggs to date.[31] Because they were vulnerable to desiccation, secretions from apocrine-like glands may have helped keep the eggs moist.[29]
According to Oftedal, early synapsids may have buried the eggs into moisture laden soil, hydrating them with contact with the moist skin, or may have carried them in a moist pouch, similar to that of monotremes (echidnas carry their eggs and offspring via a temporary pouch[32][33]), though this would limit the mobility of the parent. The latter may have been the primitive form of egg care in synapsids rather than simply burying the eggs, and the constraint on the parent's mobility would have been solved by having the eggs "parked" in nests during foraging or other activities and periodically be hydrated, allowing higher clutch sizes than could fit inside a pouch (or pouches) at once, and large eggs, which would be cumbersome to carry in a pouch, would be easier to care for. The basis of Oftedal's speculation is the fact that many species of anurans can carry eggs or tadpoles attached to the skin, or embedded within cutaneous "pouches" and how most salamanders curl around their eggs to keep them moist, both groups also having glandular skin.[31]
The glands involved in this mechanism would later evolve into true mammary glands with multiple modes of secretion in association with hair follicles. Comparative analyses of the evolutionary origin of milk constituents support a scenario in which the secretions from these glands evolved into a complex, nutrient-rich milk long before true mammals arose (with some of the constituents possibly predating the split between the synapsid and sauropsid lines). Cynodonts were almost certainly able to produce this, which allowed a progressive decline of yolk mass and thus egg size, resulting in increasingly altricial hatchlings as milk became the primary source of nutrition, which is all evidenced by the small body size, the presence of epipubic bones, and limited tooth replacement in advanced cynodonts, as well as in mammaliaforms.[29][30]
Patagia[]
Aerial locomotion first began in non-mammalian haramiyidan cynodonts, with Arboroharamiya, Xianshou, Maiopatagium and Vilevolodon both bearing exquisitely preserved, fur-covered wing membranes that stretch across the limbs and tail. Their fingers are elongated, similar to those of bats and colugos and likely sharing similar roles both as wing supports and to hang on tree branches.[34]
Within true mammals, aerial locomotion first occurs in volaticotherian eutriconodonts. Volaticotherium preserves an exquisitely preserved furry patagium with delicate wrinkles and that is very extensive, "sandwiching" the poorly preserved hands and feet and extending to the base of the tail.[35] Argentoconodon, a close relative, shares a similar femur adapted for flight stresses, indicating a similar lifestyle.[36]
Therian mammals would only achieve powered flight and gliding long after these early aeronauts became extinct, with the earliest-known gliding metatherians and bats evolving in the Paleocene.[37]
Metabolism[]
Recently, it has been found that endothermy was present as far back as the late carboniferous, with Ophiacodon. The presence of fibrolamellar, a specialised type of bone that can grow quickly while maintaining a stable structure, shows that Ophiacodon would have used its high internal body temperature to fuel a fast growth comparable to modern endotherms.[38]
Evolutionary history[]


Asaphestera, Archaeothyris and Clepsydrops, the earliest-known synapsids,[39][40] lived in the Pennsylvanian subperiod (323–299 mya) of the Carboniferous period and belonged to the series of primitive synapsids that are conventionally grouped as pelycosaurs. The pelycosaurs spread and diversified, becoming the largest terrestrial animals in the latest Carboniferous and Early Permian periods, ranging up to 6 metres (20 ft) in length. They were sprawling, bulky, possibly cold-blooded, and had small brains. Some, such as Dimetrodon, had large sails that might have helped raise their body temperature. A few relict groups lasted into the later Permian but, by the middle of the Late Permian, all of the pelycosaurs had either died off or evolved into their successors, the therapsids.[41]

The therapsids, a more advanced group of synapsids, appeared during the Middle Permian and included the largest terrestrial animals in the Middle and Late Permian. They included herbivores and carnivores, ranging from small animals the size of a rat (e.g.: Robertia), to large, bulky herbivores a ton or more in weight (e.g.: Moschops). After flourishing for many millions of years, these successful animals were all but wiped out by the Permian–Triassic mass extinction about 250 mya, the largest known extinction in Earth's history, possibly related to the Siberian Traps volcanic event.

Only a few therapsids went on to be successful in the new early Triassic landscape; they include Lystrosaurus and Cynognathus, the latter of which appeared later in the early Triassic. However, they were accompanied by the early archosaurs (soon to give rise to the dinosaurs). Some of these archosaurs, such as Euparkeria, were small and lightly built, while others, such as Erythrosuchus, were as big as or bigger than the largest therapsids.
After the Permian extinction, the synapsids did not count more than three surviving clades. The first comprised the therocephalians, which only lasted the first 20 million years of the Triassic period. The second were specialised, beaked herbivores known as dicynodonts (such as the Kannemeyeriidae), which contained some members that reached large size (up to a tonne or more). And finally there were the increasingly mammal-like carnivorous, herbivorous, and insectivorous cynodonts, including the eucynodonts from the Olenekian age, an early representative of which was Cynognathus.

Unlike the dicynodonts, which were large, the cynodonts became progressively smaller and more mammal-like as the Triassic progressed, though some forms like Trucidocynodon remained large. The first mammaliaforms evolved from the cynodonts during the early Norian age of the Late Triassic, about 225 mya.
During the evolutionary succession from early therapsid to cynodont to eucynodont to mammal, the main lower jaw bone, the dentary, replaced the adjacent bones. Thus, the lower jaw gradually became just one large bone, with several of the smaller jaw bones migrating into the inner ear and allowing sophisticated hearing.

Whether through climate change, vegetation change, ecological competition, or a combination of factors, most of the remaining large cynodonts (belonging to the Traversodontidae) and dicynodonts (of the family Kannemeyeriidae) had disappeared by the Rhaetian age, even before the Triassic–Jurassic extinction event that killed off most of the large non-dinosaurian archosaurs. The remaining Mesozoic synapsids were small, ranging from the size of a shrew to the badger-like mammal Repenomamus.
During the Jurassic and Cretaceous, the remaining non-mammalian cynodonts were small, such as Tritylodon. No cynodont grew larger than a cat. Most Jurassic and Cretaceous cynodonts were herbivorous, though some were carnivorous. The family Tritheledontidae, which first appeared near the end of the Triassic, was carnivorous and persisted well into the Middle Jurassic. The other, Tritylodontidae, first appeared at the same time as the tritheledonts, but was herbivorous. This group became extinct at the end of the Early Cretaceous epoch. Dicynodonts are thought to have become extinct near the end of the Triassic period, but there is evidence this group survived. New fossil finds have been found in the Cretaceous rocks of Gondwana.[citation needed]
Today, the 5,500 species of living synapsids, known as the mammals, include both aquatic (whales) and flying (bats) species, and the largest animal ever known to have existed (the blue whale). Humans are synapsids, as well. Most mammals are viviparous and give birth to live young rather than laying eggs with the exception being the monotremes.
Triassic and Jurassic ancestors of living mammals, along with their close relatives, had high metabolic rates. This meant consuming food (generally thought to be insects) in much greater quantity. To facilitate rapid digestion, these synapsids evolved mastication (chewing) and specialized teeth that aided chewing. Limbs also evolved to move under the body instead of to the side, allowing them to breathe more efficiently during locomotion.[42] This helped make it possible to support their higher metabolic demands.
Relationships[]
Below is a cladogram of the most commonly accepted phylogeny of synapsids, showing a long stem lineage including Mammalia and successively more basal clades such as Theriodontia, Therapsida and Sphenacodontia:[43][44]
| Synapsida |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Most uncertainty in the phylogeny of synapsids lies among the earliest members of the group, including forms traditionally placed within Pelycosauria. As one of the earliest phylogenetic analyses, Brinkman & Eberth (1983) placed the family Varanopidae with Caseasauria as the most basal offshoot of the synapsid lineage. Reisz (1986) removed Varanopidae from Caseasauria, placing it in a more derived position on the stem. While most analyses find Caseasauria to be the most basal synapsid clade, Benson's analysis (2012) placed a clade containing Ophiacodontidae and Varanopidae as the most basal synapsids, with Caseasauria occupying a more derived position. Benson attributed this revised phylogeny to the inclusion of postcranial characteristics, or features of the skeleton other than the skull, in his analysis. When only cranial or skull features were included, Caseasauria remained the most basal synapsid clade. Below is a cladogram modified from Benson's analysis (2012):[45]
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
However, more recent examination of the phylogeny of basal synapsids, incorporating newly described basal caseids and eothyridids,[46] returned Caseasauria to its position as the sister to all other synapsids. Brocklehurst et al. (2016)[46] demonstrated that many of the postcranial characters used by Benson (2012) to unite Caseasauria with Sphenacodontidae and Edaphosauridae were absent in the newly discovered postcranial material of eothyridids, and were therefore acquired convergently.
See also[]
- Anapsid
- Diapsid
- Euryapsida
- Lists of synapsids
- Mammal classification
- List of prehistoric mammals
- Timeline of the evolutionary history of life
- Vertebrate paleontology
Notes[]
- ^ Greek: συν- (syn-) "together" + ἁψίς (apsís) "arch" > *συναψίδης (synapsídes) "being with a fused arch"; synonymous with theropsids (Greek, "beast-face")
References[]
- ^ David S. Berman (2013). "Diadectomorphs, amniotes or not?". New Mexico Museum of Natural History and Science Bulletin. 60: 22–35.
- ^ Jozef Klembara; Miroslav Hain; Marcello Ruta; David S. Berman; Stephanie E. Pierce; Amy C. Henrici (2019). "Inner ear morphology of diadectomorphs and seymouriamorphs (Tetrapoda) uncovered by high‐resolution x‐ray microcomputed tomography, and the origin of the amniote crown group". Palaeontology. 63: 131–154. doi:10.1111/pala.12448.
- ^ Seeley, Harry Govier (1895). "Researches on the Structure, Organisation, and Classification of the Fossil Reptilia. Part X. On the Complete Skeleton of an Anomodont Reptile (Aristodesmus rutimeyeri, Wiedersheim), from the Bunter Sandstone of Reihen, near Basel, Giving New Evidence of the Relation of the Anomodontia to the Monotremata". Proceedings of the Royal Society of London. 59: 167–169. doi:10.1098/rspl.1895.0070.
- ^ Laurin, Michel, and Robert R. Reisz: Synapsida: Mammals and their extinct relatives. Version 14, 2011. In: The Tree of Life Web Project.
- ^ Jump up to: a b Romer, A.S. & Parsons, T.S. (1985): The Vertebrate Body. (6th ed.) Saunders, Philadelphia.
- ^ Jump up to: a b c Carroll, Robert L. (1988). Vertebrate Paleontology and Evolution. New York: W.H. Freeman & Co. ISBN 0-7167-1822-7. p. 397.
- ^ Jump up to: a b Benton, Michael J. (2005). Vertebrate Palaeontology, 3rd ed. Oxford: Blackwell Science Ltd. ISBN 0-632-05637-1. p. 122.
- ^ "Jaws to ears in the ancestors of mammals". evolution.berkeley.edu. Retrieved 2020-02-20.
- ^ "New proto-mammal fossil sheds light on evolution of earliest mammals". University of Chicago. August 7, 2013.
- ^ "Greatest mass extinction responsible for the making of modern mammals". Archived from the original on 2019-03-28. Retrieved 2015-08-22.
- ^ Angielczyk, Kenneth D. (2009). "Dimetrodon is Not a Dinosaur: Using Tree Thinking to Understand the Ancient Relatives of Mammals and their Evolution". Evolution: Education and Outreach. 2 (2): 257–271. doi:10.1007/s12052-009-0117-4. S2CID 24110810.
- ^ Modesto, S.P.; Anderson, J.S. (2004). "The phylogenetic definition of Reptilia". Systematic Biology. 53 (5): 815–821. doi:10.1080/10635150490503026. PMID 15545258.
- ^ Benton, Michael J. (2005). Vertebrate Paleontology, 3rd ed. Oxford: Blackwell Science Ltd. ISBN 0-632-05637-1. p. 120.
- ^ Angielczch, Kennenth; Kammerer, Christian F.; Frobisch, Jorg. (2013). Early Evolutionary History of Synapsida. Springer Science & Business Media. ISBN 978-94-007-6841-3, p. 11
- ^ Salentijn, L. Biology of Mineralized Tissues: Prenatal Skull Development, Columbia University College of Dental Medicine post-graduate dental lecture series, 2007
- ^ Hopson, James A. (1987). "The Mammal-Like Reptiles: A Study of Transitional Fossils". The American Biology Teacher. 49 (1): 16–26. doi:10.2307/4448410. JSTOR 4448410.
- ^ Hildebran, M.; Goslow, G. (2001). Analysis of Vertebrate Structure (5th ed.). New York: John Wiley & Sons. ISBN 0-471-29505-1.
- ^ Vickaryous, Matthew K. & Sire, Jean-Yves (2009). "The integumentary skeleton of tetrapods: origin, evolution, and development". Journal of Anatomy. 214 (4): 441–464. doi:10.1111/j.1469-7580.2008.01043.x. PMC 2736118. PMID 19422424.
- ^ Botha-Brink, J.; Modesto, S.P. (2007). "A mixed-age classed 'pelycosaur' aggregation from South Africa: earliest evidence of parental care in amniotes?". Proceedings of the Royal Society B. 274 (1627): 2829–2834. doi:10.1098/rspb.2007.0803. PMC 2288685. PMID 17848370.
- ^ Niedźwiedzki, G.; Bojanowski, M. (2012). "A Supposed Eupelycosaur Body Impression from the Early Permian of the Intra-Sudetic Basin, Poland". Ichnos. 19 (3): 150–155. doi:10.1080/10420940.2012.702549. S2CID 129567176.
- ^ Carroll, R.L. (1969). "Problems of the origin of reptiles". Biological Reviews. 44 (3): 393–432. doi:10.1111/j.1469-185X.1969.tb01218.x. S2CID 84302993.
- ^ Spindler, Frederik; Werneburg, Ralf; Schneider, Joerg W.; Luthardt, Ludwig; Annacker, Volker; Rößler, Ronny (2018). "First arboreal 'pelycosaurs' (Synapsida: Varanopidae) from the early Permian Chemnitz Fossil Lagerstätte, SE Germany, with a review of varanopid phylogeny". PalZ. 92 (2): 315–364. doi:10.1007/s12542-018-0405-9. S2CID 133846070.
- ^ Kardong, K. V. (2002). Vertebrates: Comparative Anatomy, Function, Evolution (3rd ed.). Boston: McGraw-Hill. ISBN 0-07-112235-4.
- ^ Kemp, T. S. (2006). "The origin and early radiation of the therapsid mammal-like reptiles: a palaeobiological hypothesis". Journal of Evolutionary Biology. 19 (4): 1231–1247. doi:10.1111/j.1420-9101.2005.01076.x. PMID 16780524. S2CID 3184629.
- ^ Ji, Q.; Luo, Z-X, Yuan, C-X, and Tabrum, A.R.; Yuan, Chong-Xi; Tabrum, Alan R. (February 2006). "A Swimming Mammaliaform from the Middle Jurassic and Ecomorphological Diversification of Early Mammals". Science. 311 (5764): 1123–7. Bibcode:2006Sci...311.1123J. doi:10.1126/science.1123026. PMID 16497926. S2CID 46067702.CS1 maint: multiple names: authors list (link) See also the news item at "Jurassic "Beaver" Found; Rewrites History of Mammals".
- ^ Meng, Qing-Jin; Grossnickle, David M.; Di, Liu; Zhang, Yu-Guang; Neander, April I.; Ji, Qiang; Luo, Zhe-Xi (2017). "New gliding mammaliaforms from the Jurassic". Nature. 548 (7667): 291–296. Bibcode:2017Natur.548..291M. doi:10.1038/nature23476. PMID 28792929. S2CID 205259206.
- ^ Jump up to: a b c d Ruben, J.A.; Jones, T.D. (2000). "Selective Factors Associated with the Origin of Fur and Feathers". Am. Zool. 40 (4): 585–596. doi:10.1093/icb/40.4.585.
- ^ Bajdek, Piotr; Qvarnström, Martin; Owocki, Krzysztof; Sulej, Tomasz; Sennikov, Andrey G.; Golubev, Valeriy K.; Niedźwiedzki, Grzegorz (2016). "Microbiota and food residues including possible evidence of pre-mammalian hair in Upper Permian coprolites from Russia". Lethaia. 49 (4): 455–477. doi:10.1111/let.12156.
- ^ Jump up to: a b c Oftedal, Olav T. (2002-07-01). "The mammary gland and its origin during synapsid evolution". Journal of Mammary Gland Biology and Neoplasia. 7 (3): 225–252. doi:10.1023/a:1022896515287. ISSN 1083-3021. PMID 12751889. S2CID 25806501.
- ^ Jump up to: a b Oftedal, O. T. (2012-03-01). "The evolution of milk secretion and its ancient origins". Animal. 6 (3): 355–368. doi:10.1017/S1751731111001935. ISSN 1751-732X. PMID 22436214.
- ^ Jump up to: a b Oftedal, Olav T. (2002-07-01). "The origin of lactation as a water source for parchment-shelled eggs". Journal of Mammary Gland Biology and Neoplasia. 7 (3): 253–266. doi:10.1023/A:1022848632125. ISSN 1083-3021. PMID 12751890. S2CID 8319185.
- ^ "Monotremes and marsupials". www.life.umd.edu. Retrieved 2018-08-23.
- ^ "Life History and Ecology of the Monotremata". www.ucmp.berkeley.edu. Retrieved 2018-08-23.
- ^ Luo, Zhe-Xi; Meng, Qing-Jin; Grossnickle, David M.; Di, Liu; Neander, April I.; Zhang, Yu-Guang; Ji, Qiang (2017). "New evidence for mammaliaform ear evolution and feeding adaptation in a Jurassic ecosystem". Nature. 548 (7667): 326–329. Bibcode:2017Natur.548..326L. doi:10.1038/nature23483. PMID 28792934. S2CID 4463476.
- ^ Meng, J.; Hu, Y.-M.; Wang, Y.-Q.; Wang, X.-L.; Li, C.-K. (2007). "Corrigendum: A Mesozoic gliding mammal from northeastern China". Nature. 446 (7131): 102. Bibcode:2007Natur.446Q.102M. doi:10.1038/nature05639.
- ^ Gaetano, L.C.; Rougier, G.W. (2011). "New materials of Argentoconodon fariasorum (Mammaliaformes, Triconodontidae) from the Jurassic of Argentina and its bearing on triconodont phylogeny". Journal of Vertebrate Paleontology. 31 (4): 829–843. doi:10.1080/02724634.2011.589877. S2CID 85069761.
- ^ Szalay, FS, Sargis, EJ, and Stafford, BJ (2000) Small marsupial glider from the Paleocene of Itaboraí, Brazil. Journal of Vertebrate Paleontology 20 Supplement: 73A. Presented at the Meeting of the Society of Vertebrate Paleontology.
- ^ "Ancestry of mammalian 'warm-bloodedness' revealed". www.sciencedaily.com. Society of Vertebrate Paleontology. October 29, 2015. Retrieved October 29, 2015.
- ^ Lambert, David (2001). Dinosaur Encyclopedia. ISBN 0-7894-7935-4. pp. 68–69.
- ^ Mann, Arjan; Gee, Bryan M.; Pardo, Jason D.; Marjanović, David; Adams, Gabrielle R.; Calthorpe, Ami S.; Maddin, Hillary C.; Anderson, Jason S. (2020). "Reassessment of historic 'microsaurs' from Joggins, Nova Scotia, reveals hidden diversity in the earliest amniote ecosystem". Papers in Palaeontology. Wiley. doi:10.1002/spp2.1316.
- ^ Modesto, Sean P.; Smith, Roger M. H.; Campione, Nicolás E.; Reisz, Robert R. (2011). "The last 'pelycosaur': a varanopid synapsid from the Pristerognathus Assemblage Zone, Middle Permian of South Africa". Naturwissenschaften. 98 (12): 1027–34. Bibcode:2011NW.....98.1027M. doi:10.1007/s00114-011-0856-2. PMID 22009069. S2CID 27865550.
- ^ Bramble, D. M.; Jenkins, F. A. (1993). "Mammalian locomotor-respiratory integration: Implications for diaphragmatic and pulmonary design". Science. 262 (5131): 235–240. Bibcode:1993Sci...262..235B. doi:10.1126/science.8211141. PMID 8211141.
- ^ Laurin, M.; Reisz, R.R. (2011). "Synapsida. Mammals and their extinct relatives". The Tree of Life Web Project. Retrieved 26 April 2012.
- ^ Kemp, T.S. (2011). "The origin and radiation of therapsids". In Chinsamy-Turan, A. (ed.). Forerunners of Mammals. Bloomington: Indiana University Press. pp. 3–30. ISBN 978-0-253-35697-0.
- ^ Benson, R.J. (2012). "Interrelationships of basal synapsids: cranial and postcranial morphological partitions suggest different topologies". Journal of Systematic Palaeontology. 10 (4): 601–624. doi:10.1080/14772019.2011.631042. S2CID 84706899.
- ^ Jump up to: a b Neil Brocklehurst, Robert Reisz, Vincent Fernandez, and Jörg Fröbisch (2016). "A Re-Description of 'Mycterosaurus' smithae, an Early Permian Eothyridid, and Its Impact on the Phylogeny of Pelycosaurian-Grade Synapsids". PLOS ONE. 11 (6): e0156810. Bibcode:2016PLoSO..1156810B. doi:10.1371/journal.pone.0156810. PMC 4917111. PMID 27333277.CS1 maint: uses authors parameter (link)
Further reading[]
- Colbert, E. H. (1969). Evolution of the Vertebrates (2nd ed.). New York: John Wiley & Sons Inc. ISBN 0-471-16466-6.
External links[]
| Wikispecies has information related to Synapsida. |
- Synapsida - Pelycosauria - at Palaeos
- Transitional Vertebrate Fossils - includes description of important transitional genera in the evolutionary sequence linking primitive synapsids to mammals
- Synapsids
- Tetrapod unranked clades
- Extant Pennsylvanian first appearances
- Taxa named by Henry Fairfield Osborn









