Toluene diisocyanate

| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
2,4-Diisocyanato-1-methylbenzene | |
| Other names
Tolylene diisocyanate
Methyl phenylene diisocyanate | |
| Identifiers | |
| |
3D model (JSmol)
|
|
| 744602 | |
| ChEBI |
|
| ChEMBL |
|
| ChemSpider | |
| ECHA InfoCard | 100.008.678 |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| UNII |
|
| UN number | 2078 |
CompTox Dashboard (EPA)
|
|
| Properties | |
| C9H6N2O2 | |
| Molar mass | 174.2 g/mol |
| Appearance | Colorless liquid |
| Odor | sharp, pungent[1] |
| Density | 1.214 g/cm3, liquid |
| Melting point | 21.8 °C (71.2 °F; 294.9 K) |
| Boiling point | 251 °C (484 °F; 524 K) |
| Reacts | |
| Vapor pressure | 0.01 mmHg (25°C)[1] |
| Hazards | |
| Safety data sheet | See: data page |
| GHS pictograms |    
|
| GHS Signal word | Danger |
GHS hazard statements
|
H315, H317, H318, H319, H330, H334, H335, H351, H412 |
| P201, P202, P260, P261, P264, P271, P272, P273, P280, P281, P284, P285, P302+352, P304+340, P304+341, P305+351+338, P308+313, P310, P312, P320, P321, P332+313, P333+313, P337+313, P342+311 | |
| NFPA 704 (fire diamond) | 
3
1
1 |
| Flash point | 127 °C (261 °F; 400 K) |
| Explosive limits | 0.9–9.5%[1] |
| Lethal dose or concentration (LD, LC): | |
LC50 (median concentration)
|
14 ppm (rat, 4 hr) 13.9 ppm (guinea pig, 4 hr) 9.7 ppm (mouse, 4 hr) 11 ppm (rabbit, 4 hr)[2] |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
C 0.02 ppm (0.14 mg/m3)[1] |
REL (Recommended)
|
Ca[1] |
IDLH (Immediate danger)
|
Ca [2.5 ppm][1] |
| Related compounds | |
Related isocyanates
|
Methylene diphenyl diisocyanate |
Related compounds
|
Polyurethane |
| Supplementary data page | |
Structure and
properties |
Refractive index (n), Dielectric constant (εr), etc. |
Thermodynamic
data |
Phase behaviour solid–liquid–gas |
Spectral data
|
UV, IR, NMR, MS |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Toluene diisocyanate (TDI) is an organic compound with the formula CH3C6H3(NCO)2. Two of the six possible isomers are commercially important: 2,4-TDI (CAS: 584-84-9) and 2,6-TDI (CAS: 91-08-7). 2,4-TDI is produced in the pure state, but TDI is often marketed as 80/20 and 65/35 mixtures of the 2,4 and 2,6 isomers respectively. It is produced on a large scale, accounting for 34.1% of the global isocyanate market in 2000, second only to MDI.[3] Approximately 1.4 billion kilograms were produced in 2000.[4] All isomers of TDI are colorless, although commercial samples can appear yellow.
Synthesis[]
2,4-TDI is prepared in three steps from toluene via dinitrotoluene and 2,4-diaminotoluene (TDA). Finally, the TDA is subjected to phosgenation, i.e., treatment with phosgene to form TDI. This final step produces HCl as a byproduct and is a major source of industrial hydrochloric acid.[4]
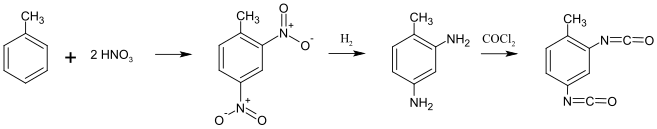
Distillation of the raw TDI mixture produces an 80:20 mixture of 2,4-TDI and 2,6-TDI, known as TDI (80/20). Differentiation or separation of the TDI (80/20) can be used to produce pure 2,4-TDI and a 65:35 mixture of 2,4-TDI and 2,6-TDI, known as TDI (65/35).
Applications[]
The isocyanate functional groups in TDI react with hydroxyl groups to form carbamate (urethane) links. The two isocyanate groups in TDI react at different rates: The 4-position is approximately four times more reactive than the 2-position. 2,6-TDI is a symmetrical molecule and thus has two isocyanate groups of similar reactivity, similar to the 2-position on 2,4-TDI. However, since both isocyanate groups are attached to the same aromatic ring, reaction of one isocyanate group will cause a change in the reactivity of the second isocyanate group.[3] It is also sometimes used in rocket propellants.[5]
It is used in the production of rigid polyurethane foams with a high temperature stability.
Hazards[]
The LD50 for TDI is 5800 mg/kg for oral contact and LC50 of 610 mg/m3 for the vapour. Despite the indicated low toxicity, TDI is classified as “very toxic” by the European Community.[4]
In the United States, the Occupational Safety and Health Administration has set a permissible exposure limit with a ceiling at 0.02 ppm (0.14 mg/m3), while the National Institute for Occupational Safety and Health has not established a recommended exposure limit, due to the classification of toluene diisocyanate as a possible occupational carcinogen.[6] This chemical was one of many that caused two massive explosions in a chemical warehouse stationed in Tianjin, China on August 13, 2015.[7]
Information is available on handling, personal protective equipment, exposure monitoring, transport, storage, sampling and analysis of TDI, dealing with accidents, and health and environmental themes.[8] All major producers of TDI are members of the International Isocyanate Institute,[citation needed] whose aim is the promotion of the safe handling of TDI in the workplace, community, and environment.
High-level exposure can result in reactive airways dysfunction syndrome.[citation needed]
See also[]
References[]
- ^ Jump up to: a b c d e f NIOSH Pocket Guide to Chemical Hazards. "#0621". National Institute for Occupational Safety and Health (NIOSH).
- ^ "Toluene-2,4-diisocyanate". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- ^ Jump up to: a b Randall, D.; Lee, S. (2003). The Polyurethanes Book. New York: Wiley. ISBN 978-0-470-85041-1.
- ^ Jump up to: a b c Six, C.; Richter, F. "Isocyanates, Organic". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a14_611.CS1 maint: multiple names: authors list (link)
- ^ "Ababil-100/Al Fat'h". GlobalSecurity.org. Archived from the original on 15 April 2019.
- ^ National Institute for Occupational Safety and Health (May 1994). "Documentation for Immediately Dangerous To Life or Health Concentrations (IDLHs)". Centers for Disease Control and Prevention.
- ^ CNN
- ^ Allport, D. C.; Gilbert, D. S.; Outterside, S. M., eds. (2003). MDI and TDI: Safety, Health and the Environment: A Source Book and Practical Guide. Wiley. ISBN 978-0-471-95812-3.
External links[]
- International Chemical Safety Card 0339
- IARC Monograph: "Toluene Diisocyanates"
- NIOSH Pocket Guide to Chemical Hazards
- NIOSH Safety and Health Topic: Isocyanates, from the website of the National Institute for Occupational Safety and Health (NIOSH)
- International Isocyanate Institute http://www.diisocyanates.org
- Isocyanates
- Monomers
- IARC Group 2B carcinogens
- Alkyl-substituted benzenes