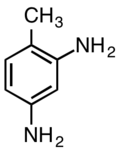
2,4-Diaminotoluene
| |
| Names | |
|---|---|
| Preferred IUPAC name
4-Methylbenzene-1,3-diamine | |
| Other names
2,4-Toluenediamine
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.002.231 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| Properties | |
| C7H10N2 | |
| Molar mass | 122.171 g·mol−1 |
| Appearance | White solid |
| Density | 1.521 g/cm3 |
| Melting point | 97 to 99 °C (207 to 210 °F; 370 to 372 K) |
| Boiling point | 283 to 285 °C (541 to 545 °F; 556 to 558 K) |
| Hazards | |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
none[1] |
REL (Recommended)
|
Ca[1] |
IDLH (Immediate danger)
|
Ca [N.D.][1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
2,4-Diaminotoluene is an organic compound with the formula C6H3(NH2)2CH3. It is one isomer of six with this formula. It is a white solid although commercial samples are often yellow-tan.
Preparation[]
It is prepared by hydrogenation of 2,4-dinitrotoluene using a nickel catalyst. Commercial samples often contain up to 20% of the 2,6-isomer.[2]
A laboratory method involves reduction of 2,4-dinitrotoluene with iron powder.[3]
Use[]
It is mainly used as a precursor to toluene diisocyanate, a precursor to polyurethane.
Its reaction with benzenediazonium chloride gives the cationic azo dye Basic Orange 1. Condensation of 2,4-diaminotoluene with acetaldehyde gives the acridine dye called Basic Yellow 9.[4]

Synthesis of C.I. Basic Yellow 9, an acridine dye.
References[]
- ^ a b c NIOSH Pocket Guide to Chemical Hazards. "#0620". National Institute for Occupational Safety and Health (NIOSH).
- ^ Robert A. Smiley "Phenylene- and Toluenediamines" in Ullmann's Encyclopedia of Industrial Chemistry, 2002, Wiley-VCH, Weinheim. doi:10.1002/14356007.a19_405
- ^ "2,4-DIAMINOTOLUENE". Organic Syntheses. 11: 32. 1931. doi:10.15227/orgsyn.011.0032.
- ^ Thomas Gessner and Udo Mayer "Triarylmethane and Diarylmethane Dyes" in Ullmann's Encyclopedia of Industrial Chemistry 2002, Wiley-VCH, Weinheim.doi:10.1002/14356007.a27_179
Categories:
- Anilines
- Diamines
- Monomers
- Alkyl-substituted benzenes