Triacetic acid lactone

| |
| Names | |
|---|---|
| Preferred IUPAC name
4-Hydroxy-6-methyl-2H-pyran-2-one | |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.010.564 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C6H6O3 | |
| Molar mass | 126.12 g mol−1 |
| Appearance | light yellow crystal powder |
| Density | 1.348 g cm−3 |
| Melting point | 188 to 190 °C (370 to 374 °F; 461 to 463 K) |
| Boiling point | 285.9 °C (546.6 °F; 559.0 K) |
| 8.60 g L-1 at 20°C in H2O | |
| Hazards | |
| Main hazards | Moderately Toxic |
| GHS labelling: | |

| |
Signal word
|
Warning |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P403+P233, P405, P501 | |
| Flash point | 127.9 °C (262.2 °F; 401.0 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Triacetic acid lactone (TAL;[1] 4-hydroxy-6-methyl-2-pyrone) is an organic compound derived enzymatically from glucose. It is a light yellow solid that is soluble in organic solvents.
Structure[]
Triacetic acid lactone consists of two main tautomers.

The tautomer on the left, featuring a 4-hydroxy group, the C4 carbon, is dominant. Triacetic acid lactone is classified as a 2-pyrone compound owing to the ketone group on the C2 carbon in its dominant form.
Synthesis[]
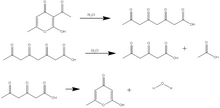
Triacetic acid lactone is synthesized either from dehydroacetic acid, another 2-pyrone derivative, or from glucose by enzymatic catalysis. In its original synthesis, triacetic acid lactone was obtained by treatment of dehydroacetic acid with sulfuric acid at 135 °C. Dehydroacetic acid undergoes ring-opening and hydration to form "tetracetic acid".[2] Upon cooling, triacetic acid reverts to a lactone ring similar to the dehydroacetic acid structure, and the triacetic acid lactone is recovered by crystallization in cold water.
Biosynthesis[]
The microbial synthesis of triacetic acid lactone requires the enzyme (2-PS).[3] This enzyme has been examined in two hosts Escherichia coli and Saccharomyces cerevisiae. The Saccharomyces cerevisiae host being used during the synthesis produces a higher yield (70%) compared with the Escherichia coli host, which produces a yield of 40% of triacetic acid lactone. This enzyme catalyzes the synthesis of triacetic acid lactone from acetyl-CoA via two subsequent condensations with malonyl-CoA. This produces an intermediate of 3,5-diketohexanoate thioester, which undergoes ring closure to produce triacetic acid lactone.
Reactivity[]
The lactone is a versatile intermediate in organic synthesis.[4] It has also been described as a platform chemical, meaning that it could be the precursor to other fine chemicals. The lactone undergoes decarboxylation to acetylacetone. It is also a precursor to sorbic acid, , and . Dienoic acid is used to inhibit the growth of various molds and hexenoic acid is used as a flavoring agent.[5] Acetylacetone is used for metal extraction and plating and as a food additive.[6]
References[]
- ^ "New Sustainable Production Method Could Advance Plastics and Pharmaceuticals" (Press release). University of Texas. 13 February 2018 – via Drug Discovery & Development Magazine.
- ^ Collie, J. Norman (1891). "LVI.?The lactone of triacetic acid". Journal of the Chemical Society, Transactions. 59: 607–617. doi:10.1039/CT8915900607.
- ^ Xie, Dongming; Shao, Zengyi; Achkar, Jihane; Zha, Wenjuan; Frost, John W.; Zhao, Huimin (2006). "Microbial synthesis of triacetic acid lactone". Biotechnology and Bioengineering. 93 (4): 727–36. doi:10.1002/bit.20759. PMID 16245348. S2CID 2626483.
- ^ Moreno-Mañas, Marcial; Pleixats, Roser (1992). "Dehydroacetic Acid, Triacetic Acid Lactone, and Related Pyrones". Advances in Heterocyclic Chemistry. 53: 1–84. doi:10.1016/S0065-2725(08)60861-2. ISBN 9780120207534.
- ^ Jacoby, Mitch (2012). "Teaming Up for Biobased Chemicals". Chem. Eng. News. 90 (32): 37–38. doi:10.1021/cen-09032-scitech1.
- ^ Chia, Mei; Schwartz, Thomas J.; Shanks, Brent H.; Dumesic, James A. (2012). "Triacetic acid lactone as a potential biorenewable platform chemical". Green Chemistry. 14 (7): 1850. doi:10.1039/C2GC35343A.
- 2-Pyrones
- Phenols