Sorbic acid
| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
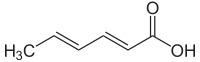
(2E,4E)-Hexa-2,4-dienoic acid | |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.003.427 |
| E number | E200 (preservatives) |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| Properties | |
| C6H8O2 | |
| Molar mass | 112.128 g·mol−1 |
| Density | 1.204 g/cm3 |
| Melting point | 135 °C (275 °F; 408 K) |
| Boiling point | 228 °C (442 °F; 501 K) |
| 1.6 g/L at 20 °C | |
| Acidity (pKa) | 4.76 at 25 °C |
| Hazards | |
| NFPA 704 (fire diamond) | 
2
1
0 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Sorbic acid, or 2,4-hexadienoic acid, is a natural organic compound used as a food preservative.[1] It has the chemical formula CH3(CH)4CO2H. It is a colourless solid that is slightly soluble in water and sublimes readily. It was first isolated from the unripe berries of the Sorbus aucuparia (rowan tree), hence its name.
Production[]
The traditional route to sorbic acid involves condensation of malonic acid and trans-butenal.[2] It can also be prepared from isomeric hexadienoic acids, which are available via a nickel-catalyzed reaction of allyl chloride, acetylene, and carbon monoxide. The route used commercially, however, is from crotonaldehyde and ketene.[3] An estimated 30,000 tons are produced annually.[4]
History[]
Sorbic acid was isolated in 1859 by distillation of rowanberry oil by A. W. von Hofmann.[5] This affords parasorbic acid, the lactone of sorbic acid, which he converted to sorbic acid by hydrolysis. Its antimicrobial activities were discovered in the late 1930s and 1940s, and it became commercially available in the late 1940s and 1950s. Beginning in the 1980s, sorbic acid and its salts were used as inhibitors of Clostridium botulinum in meat products to replace the use of nitrites, which can produce carcinogenic nitrosamines.[6]
Properties and uses[]
With a pKa of 4.76, it is about as acidic as acetic acid.
Sorbic acid and its salts, such as sodium sorbate, potassium sorbate, and calcium sorbate, are antimicrobial agents often used as preservatives in food and drinks to prevent the growth of mold, yeast, and fungi. In general the salts are preferred over the acid form because they are more soluble in water, but the active form is the acid. The optimal pH for the antimicrobial activity is below pH 6.5. Sorbates are generally used at concentrations of 0.025% to 0.10%. Adding sorbate salts to food will, however, raise the pH of the food slightly so the pH may need to be adjusted to assure safety. It is found in foods such as cheeses and breads.
The E numbers are:
- E200 Sorbic acid
- E201 Sodium sorbate
- E202 Potassium sorbate
- E203 Calcium sorbate
Some molds (notably some Trichoderma and Penicillium strains) and yeasts are able to detoxify sorbates by decarboxylation, producing trans-1,3-pentadiene. The pentadiene manifests as a typical odor of kerosene or petroleum. Other detoxification reactions include reduction to 4-hexenol and 4-hexenoic acid.[7]
Sorbic acid can also be used as an additive for cold rubber, and as an intermediate in the manufacture of some plasticizers and lubricants.[8]
Safety[]
This section needs additional citations for verification. (January 2018) |
The LD50 value of sorbic acid is estimated to be between 7.4 and 10 g/kg. Sorbic acid and sorbates therefore have a very low mammalian toxicity – hence their extensive use in food and beverage preservation. Sorbic acid occurs naturally in wild berries, is relatively unstable and rapidly degraded in soil, hence it is considered environmentally friendly. In the body it is generally metabolized by the same oxidation pathway as the 5-carbon saturated fatty acid caproic acid. There is a general consensus that sorbic acid and sorbates are intrinsically devoid of carcinogenic activity. However they have been shown to have the potential to undergo conversion to potential mutagens either through oxidation, or through a chemical reaction with nitrites at pH of 2-4.2 – the latter conditions being ones that mimic the gastric environment. In living yeast cells sorbic acid enhances oxygen free radical formation by the mitochondrial electron transport chain, leading to potential damage to the mitochondrial DNA.
See also[]
References[]
- ^ Piper JD, Piper PW (2017). "Benzoate and Sorbate Salts: A Systematic Review of the Potential Hazards of These Invaluable Preservatives and the Expanding Spectrum of Clinical Uses for Sodium Benzoate". Comprehensive Reviews in Food Science and Food Safety. 16 (5): 868–880. doi:10.1111/1541-4337.12284.
- ^ C. F. H. Allen, J. VanAllan (1944). "Sorbic Acid". Org. Synth. 24: 92. doi:10.15227/orgsyn.024.0092.CS1 maint: uses authors parameter (link)
- ^ Ashford's Dictionary of Industrial Chemicals, Third edition, 2011, page 8482
- ^ Erich Lück, Martin Jager and Nico Raczek "Sorbic Acid" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2000.doi:10.1002/14356007.a24_507
- ^ Hofmann, A.W. (1859). "Neue flüchtige Säure der Vogelbeeren" [New volatile acid of rowan berries]. Annalen der Chemie und Pharmacie (in German). 110 (2): 129–140. Hofmann named sorbic acid on p. 133: "Ich schlage für die krystallinische Säure den Namen Sorbinsäure vor, wodurch ein alter Name der in den Vogelbeeren gefundenen Aepfelsäure neue Bedeutung gewinnt." (For the crystalline acid, I suggest the name "sorbic acid", whereby an old name of the malic acid that's found in rowan berries gains new meaning.)
- ^ A. S. Naidu, ed. (2000). Natural food antimicrobial systems. p. 637. ISBN 0-8493-2047-X.
- ^ Kinderlerer JL, Hatton PV (1990). "Fungal metabolites of sorbic acid". Food Addit Contam. 7 (5): 657–69. doi:10.1080/02652039009373931. PMID 2253810.
- ^ Bingham E, Cohrssen B (2012). Patty's Toxicology. John Wiley & Sons. p. 547.
External links[]
- Enoic acids
- Preservatives
- E-number additives
- Conjugated dienes