Trimethylaluminium
| |

| |
| Names | |
|---|---|
| IUPAC name
Trimethylalumane
| |
| Other names
Trimethylaluminum; aluminium trimethyl; aluminum trimethyl
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.000.776 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| Properties | |
| C6H18Al2 | |
| Molar mass | 144.17 g/mol 72.09 g/mol (C3H9Al) |
| Appearance | Colorless liquid |
| Density | 0.752 g/cm3 |
| Melting point | 15 °C (59 °F; 288 K) |
| Boiling point | 125–130 °C (257–266 °F; 398–403 K) [1][2] |
| Reacts | |
| Vapor pressure |
|
| Viscosity |
|
| Thermochemistry | |
Heat capacity (C)
|
155.6 J/mol·K[2] |
Std molar
entropy (S |
209.4 J/mol·K[2] |
Std enthalpy of
formation (ΔfH⦵298) |
−136.4 kJ/mol[2] |
Gibbs free energy (ΔfG˚)
|
−9.9 kJ/mol[2] |
| Hazards | |
| Main hazards | Pyrophoric |
| GHS pictograms |   [1] [1]
|
| GHS Signal word | Danger |
GHS hazard statements
|
H250, H260, H314[1] |
| P222, P223, P231+232, P280, P370+378, P422[1] | |
| NFPA 704 (fire diamond) | 
3
4
3 |
| Flash point | −17.0 °C (1.4 °F; 256.1 K) [1] |
| Related compounds | |
Related compounds
|
Triethylaluminium |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Trimethylaluminium is one of the simplest examples of an organoaluminium compound. Despite its name it has the formula Al2(CH3)6 (abbreviated as Al2Me6 or TMA), as it exists as a dimer. This colorless liquid is pyrophoric. It is an industrially important compound, closely related to triethylaluminium.[3]
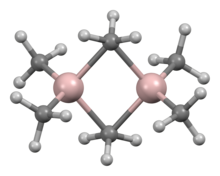
Structure and bonding[]
The structure and bonding in Al2R6 and diborane are analogous (R = alkyl). In Al2Me6, the Al-C(terminal) and Al-C(bridging) distances are 1.97 and 2.14 Å, respectively. The Al center is tetrahedral.[4] The carbon atoms of the bridging methyl groups are each surrounded by five neighbors: three hydrogen atoms and two aluminium atoms. The methyl groups interchange readily intramolecularly. At higher temperatures, the dimer cracks into monomeric AlMe3.[5]
Synthesis[]
TMA is prepared via a two-step process that can be summarized as follows:
- 2 Al + 6 CH3Cl + 6 Na → Al2(CH3)6 + 6 NaCl
Applications[]
Catalysis[]
Starting with the invention of Ziegler-Natta catalysis, organoaluminium compounds have a prominent role in the production of polyolefins, such as polyethylene and polypropylene. Methylaluminoxane, which is produced from TMA, is an activator for many transition metal catalysts.
Semiconductor applications[]
TMA is also used in semiconductor fabrication to deposit thin film, high-k dielectrics such as Al2O3 via the processes of chemical vapor deposition or atomic layer deposition. TMA is the preferred precursor for metalorganic vapour phase epitaxy (MOVPE) of aluminium-containing compound semiconductors, such as AlAs, AlN, AlP, AlSb, AlGaAs, , , AlGaN, , , etc. Criteria for TMA quality focus on (a) elemental impurities, (b) oxygenated and organic impurities.
Photovoltaic applications[]
In deposition processes very similar to semiconductor processing, TMA is used to deposit thin film, low-k (non-absorbing) dielectric layer stacks with Al2O3 via the processes of chemical vapor deposition or atomic layer deposition. The Al2O3 provides excellent surface passivation of p-doped silicon surfaces. The Al2O3 layer is typically the bottom layer with multiple silicon nitride (SixNy) layers for capping.
Reactions[]
[]
Trimethylaluminium is hydrolyzed readily, even dangerously:
- AlMe3 + 1.5 H2O → 0.5 Al2O3 + 3 CH4
Under controlled conditions, the reaction can be stopped to give methylaluminoxane:
- AlMe3 + H2O → 1/n [AlMeO]n + 2 CH4
Alcoholysis and aminolysis reactions proceed comparably. For example, dimethylamine gives the dialuminium diamide dimer:[6]
- 2 AlMe3 + 2 HNMe2 → [AlMe2NMe2]2 + 2 CH4
Reactions with metal chlorides[]
TMA reacts with many metal halides to install alkyl groups. When combined with gallium trichloride, it gives trimethylgallium.[7] Al2Me6 reacts with aluminium trichloride to give (AlMe2Cl)2.
TMA/metal halide reactions have emerged as reagents in organic synthesis. Tebbe's reagent, which is used for the methylenation of esters and ketones, is prepared from TMA and titanocene dichloride.[8] In combination with 20 to 100 mol % Cp2ZrCl2 (zirconocene dichloride), the (CH3)2Al-CH3 adds "across" alkynes to give vinyl aluminum species that are useful in organic synthesis in a reaction known as carboalumination.[9]
Adducts[]
As for other "electron-deficient" compounds, trimethylaluminium gives adducts R3N.AlMe3. The Lewis acid properties of AlMe3 have been quantified.[10] The enthalpy data show that AlMe3 is a hard acid and its acid parameters in the ECW model are EA =8.66 and CA = 3.68.
These adducts, e.g. the complex with the tertiary amine DABCO, are safer to handle than TMA itself.[11]
The NASA ATREX mission (Anomalous Transport Rocket Experiment) employed the white smoke that TMA forms on air contact to study the high altitude jet stream.
Synthetic reagent[]
TMA is a source of methyl nucleophiles, akin to methyl lithium, but less reactive. It reacts with ketones to give, after a hydrolytic workup, tertiary alcohols.
Safety[]
Trimethylaluminium is pyrophoric, reacting violently with air and water.
References[]
- ^ Jump up to: a b c d e f Sigma-Aldrich Co., Trimethylaluminum. Retrieved on 2014-05-05.
- ^ Jump up to: a b c d e http://chemister.ru/Database/properties-en.php?dbid=1&id=3290
- ^ Krause, Michael J.; Orlandi, Frank; Saurage, Alfred T.; Zietz, Joseph R. (2000). "Aluminum Compounds, Organic". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a01_543.
- ^ Holleman, A. F.; Wiberg, E. (2001). Inorganic Chemistry. San Diego: Academic Press. ISBN 0-12-352651-5.
- ^ Vass, Gábor; Tarczay, György; Magyarfalvi, Gábor; Bödi, András; Szepes, László (2002). "HeI Photoelectron Spectroscopy of Trialkylaluminum and Dialkylaluminum Hydride Compounds and Their Oligomers". Organometallics. 21 (13): 2751–2757. doi:10.1021/om010994h.
- ^ Lipton, Michael F.; Basha, Anwer; Weinreb, Steven M. (1979). "Conversion of Esters to Amides with Dimethylaluminum Amides: N,N-Dimethylcyclohexanecarboxamide". Organic Syntheses. 59: 49. doi:10.15227/orgsyn.059.0049.
- ^ Gaines, D. F.; Borlin, Jorjan; Fody, E. P. (1974). "Trimethylgallium". Inorganic Syntheses. 15: 203–207. doi:10.1002/9780470132463.ch45.
- ^ Pine, S. H.; Kim, V.; Lee, V. (1990). "Enol ethers by methylenation of esters: 1-Phenoxy-1-phenylethene and 3,4-dihydro-2-methylene-2H-1-benzopyran". Org. Synth. 69: 72. doi:10.15227/orgsyn.069.0072.
- ^ Negishi, E.; Matsushita, H. (1984). "Palladium-Catalyzed Synthesis of 1,4-Dienes by Allylation of Alkenyalane: α-Farnesene [1,3,6,10-Dodecatetraene, 3,7,11-trimethyl-]". Organic Syntheses. 62: 31. doi:10.15227/orgsyn.062.0031.CS1 maint: multiple names: authors list (link)
- ^ Henrickson, C. H.; Duffy, D.; Eyman, D. P. (1968). "Lewis acidity of Alanes. Interactions of Trimethylalane with Amines, Ethers, and Phosphines". Inorganic Chemistry. 7 (6): 1047–1051. doi:10.1021/ic50064a001.
- ^ Vinogradov, Andrej; Woodward, S. (2010). "Palladium-Catalyzed Cross-Coupling Using an Air-Stable Trimethylaluminum Source. Preparation of Ethyl 4-Methylbenzoate". Organic Syntheses. 87: 104. doi:10.15227/orgsyn.087.0104.
External links[]
| Wikimedia Commons has media related to Trimethylaluminium. |
- Organoaluminium compounds
- Coordination compounds