Aluminium hydride

| |
| Names | |
|---|---|
| Preferred IUPAC name
Aluminium hydride | |
| Systematic IUPAC name
Alumane | |
| Other names
Alane
Aluminic hydride | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.029.139 |
| 245 | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| Properties | |
| AlH3 | |
| Molar mass | 29.99 g/mol |
| Appearance | white crystalline solid, non-volatile, highly polymerized, needle-like crystals |
| Density | 1.477 g/cm3, solid |
| Melting point | 150 °C (302 °F; 423 K) starts decomposing at 105 °C (221 °F) |
| reacts | |
| Solubility | soluble in ether reacts in ethanol |
| Thermochemistry | |
Heat capacity (C)
|
40.2 J/mol K |
Std molar
entropy (S |
30 J/mol K |
Std enthalpy of
formation (ΔfH⦵298) |
-11.4 kJ/mol |
Gibbs free energy (ΔfG˚)
|
46.4 kJ/mol |
| Related compounds | |
Related compounds
|
Lithium aluminium hydride, diborane |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Aluminium hydride (also known as alane or alumane) is an inorganic compound with the formula AlH3. It presents as a white solid and may be tinted grey with decreasing particle size and impurity levels. Depending upon synthesis conditions, the surface of the alane may be passivated with a thin layer of aluminum oxide and/or hydroxide. Alane and its derivatives are used as reducing agents in organic synthesis.[1]
Structure[]
Alane is a polymer. Hence, its formula is sometimes represented with the formula (AlH3)n. Alane forms numerous polymorphs, which are named α-alane, α’-alane, β-alane, γ-alane, δ-alane, ε-alane and ζ-alane. α-Alane has a cubic or rhombohedral morphology, whereas α’-alane forms needle-like crystals and γ-alane forms a bundle of fused needles. Alane is soluble in THF and ether. The rate of the precipitation of solid alane from ether varies with the preparation method.[2]
The crystal structure of α-alane has been determined and features aluminium atoms surrounded by 6 hydrogen atoms that bridge to 6 other aluminium atoms. The Al-H distances are all equivalent (172pm) and the Al-H-Al angle is 141°.[3]
 |
 |

|
| α-AlH3 unit cell | Al coordination | H coordination |
α-Alane is the most thermally stable polymorph. β-alane and γ-alane are produced together, and convert to α-alane upon heating. δ, ε, and θ-alane are produced in still other crystallization conditions. Although they are less thermally stable, δ, ε, and θ polymorphs do not convert into α-alane upon heating.[2]
Molecular forms of alane[]
Monomeric AlH3 has been isolated at low temperature in a solid noble gas matrix and shown to be planar.[4] The dimer Al2H6 has been isolated in solid hydrogen. It is isostructural with diborane (B2H6) and digallane (Ga2H6).[5][6]
Preparation[]
Aluminium hydrides and various complexes thereof have long been known.[7] Its first synthesis was published in 1947, and a patent for the synthesis was assigned in 1999.[8][9] Aluminium hydride is prepared by treating lithium aluminium hydride with aluminium trichloride.[10] The procedure is intricate: attention must be given to the removal of lithium chloride.
- 3 LiAlH4 + AlCl3 → 4 AlH3 + 3 LiCl
The ether solution of alane requires immediate use, because polymeric material rapidly precipitates as a solid. Aluminium hydride solutions are known to degrade after 3 days. Aluminium hydride is more reactive than LiAlH4.[2]
Several other methods exist for the preparation of aluminium hydride:
- 2 LiAlH4 + BeCl2 → 2 AlH3 + Li2BeH2Cl2
- 2 LiAlH4 + H2SO4 → 2 AlH3 + Li2SO4 + 2 H2
- 2 LiAlH4 + ZnCl2 → 2 AlH3 + 2 LiCl + ZnH2
- 2 LiAlH4 + I2 → 2 AlH3 + 2 LiI + H2
Electrochemical synthesis[]
Several groups have shown that alane can be produced electrochemically.[11][12][13][14][15] Different electrochemical alane production methods have been patented.[16][17] Electrochemically generating alane avoids chloride impurities. Two possible mechanisms are discussed for the formation of alane in Clasen's electrochemical cell containing THF as the solvent, sodium aluminium hydride as the electrolyte, an aluminium anode, and an iron (Fe) wire submerged in mercury (Hg) as the cathode. The sodium forms an amalgam with the Hg cathode preventing side reactions and the hydrogen produced in the first reaction could be captured and reacted back with the sodium mercury amalgam to produce sodium hydride. Clasen's system results in no loss of starting material. For insoluble anodes, reaction 1 occurs, while for soluble anodes, anodic dissolution is expected according to reaction 2:
1. AlH4− - e− → AlH3 · nTHF + ½H2
2. 3AlH4− + Al - 3e− → 4AlH3 · nTHF
In reaction 2, the aluminium anode is consumed, limiting the production of aluminium hydride for a given electrochemical cell.
The crystallization and recovery of aluminum hydride from electrochemically generated alane has been demonstrated.[14][15]
High pressure hydrogenation of aluminium metal[]
α-AlH3 can be produced by hydrogenation of aluminium metal at 10GPa and 600 °C (1,112 °F). The reaction between the liquified hydrogen produces α-AlH3 which could be recovered under ambient conditions.[18]
Reactions[]
Formation of adducts with Lewis bases[]
AlH3 readily forms adducts with strong Lewis bases. For example, both 1:1 and 1:2 complexes form with trimethylamine. The 1:1 complex is tetrahedral in the gas phase,[19] but in the solid phase it is dimeric with bridging hydrogen centres, (NMe3Al(μ-H))2.[20] The 1:2 complex adopts a trigonal bipyramidal structure.[19] Some adducts (e.g. dimethylethylamine alane, NMe2Et · AlH3) thermally decompose to give aluminium metal and may have use in MOCVD applications.[21]
Its complex with diethyl ether forms according to the following stoichiometry:
- AlH3 + (C2H5)2O → H3Al · O(C2H5)2
The reaction with lithium hydride in ether produces lithium aluminium hydride:
- AlH3 + LiH → LiAlH4
Reduction of functional groups[]
In organic chemistry, aluminium hydride is mainly used for the reduction of functional groups.[22] In many ways, the reactivity of aluminium hydride is similar to that of lithium aluminium hydride. Aluminium hydride will reduce aldehydes, ketones, carboxylic acids, anhydrides, acid chlorides, esters, and lactones to their corresponding alcohols. Amides, nitriles, and oximes are reduced to their corresponding amines.
In terms of functional group selectivity, alane differs from other hydride reagents. For example, in the following cyclohexanone reduction, lithium aluminium hydride gives a trans:cis ratio of 1.9 : 1, whereas aluminium hydride gives a trans:cis ratio of 7.3 : 1.[23]

Alane enables the hydroxymethylation of certain ketones (that is the replacement of C-H by C-CH2OH at the alpha position).[24] The ketone itself is not reduced as it is "protected" as its enolate.
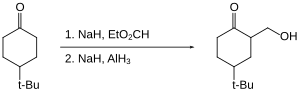
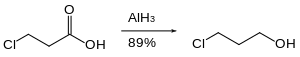
Organohalides are reduced slowly or not at all by aluminium hydride. Therefore, reactive functional groups such as carboxylic acids can be reduced in the presence of halides.[25]
Nitro groups are not reduced by aluminium hydride. Likewise, aluminium hydride can accomplish the reduction of an ester in the presence of nitro groups.[26]

Aluminium hydride can be used in the reduction of acetals to half protected diols.[27]

Aluminium hydride can also be used in epoxide ring opening reaction as shown below.[28]

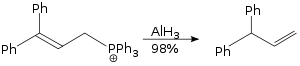
The allylic rearrangement reaction carried out using aluminium hydride is a SN2 reaction, and it is not sterically demanding.[29]
Aluminium hydride even reduces carbon dioxide to methane under heating:
- 4 AlH3 + 3 CO2 → 3 CH4 + 2 Al2O3
Hydroalumination[]
Aluminium hydride has been shown to add to propargylic alcohols.[30] Used together with titanium tetrachloride, aluminium hydride can add across double bonds.[31] Hydroboration is a similar reaction.
Fuel[]
In its passivated form, Alane is an active candidate for storing hydrogen, and can be used for efficient power generation via fuel cell applications, including fuel cell and electric vehicles and other lightweight power applications. AlH3 contains up to 10% hydrogen by weight, corresponding to 148g H2/L, or twice the hydrogen density of liquid H2. In its unpassivated form, alane is also a promising rocket fuel additive, capable of delivering impulse efficiency gains of up to 10%.[32]
Precautions[]
Alane is not spontaneously flammable. It should be handled similarly to that of other complex metal hydride reducing agents like lithium aluminium hydride. Alane will decompose in air and water, although passivation greatly diminishes decomposition rate. Passivated alane is generally assigned a hazard classification of 4.3 (chemicals which in contact with water, emit flammable gases).[33]
References[]
- ^ Brown, H. C.; Krishnamurthy, S. (1979). "Forty Years of Hydride Reductions". Tetrahedron. 35 (5): 567–607. doi:10.1016/0040-4020(79)87003-9.[verification needed]
- ^ Jump up to: a b c US application 2007066839, Lund, G. K.; Hanks, J. M.; Johnston, H. E., "Method for the Production of α-Alane"
- ^ Turley, J. W.; Rinn, H. W. (1969). "The Crystal Structure of Aluminum Hydride". Inorganic Chemistry. 8 (1): 18–22. doi:10.1021/ic50071a005.
- ^ Kurth, F. A.; Eberlein, R. A.; Schnöckel, H.-G.; Downs, A. J.; Pulham, C. R. (1993). "Molecular Aluminium Trihydride, AlH3: Generation in a Solid Noble Gas Matrix and Characterisation by its Infrared Spectrum and ab initio Calculations". Journal of the Chemical Society, Chemical Communications. 1993 (16): 1302–1304. doi:10.1039/C39930001302.
- ^ Andrews, L.; Wang, X. (2003). "The Infrared Spectrum of Al2H6 in Solid Hydrogen". Science. 299 (5615): 2049–2052. Bibcode:2003Sci...299.2049A. doi:10.1126/science.1082456. PMID 12663923. S2CID 45856199.
- ^ Pulham, C. R.; Downs, A. J.; Goode, M. J.; Rankin D. W. H.; Robertson, H. E. (1991). "Gallane: Synthesis, Physical and Chemical Properties, and Structure of the Gaseous Molecule Ga2H6 as Determined by Electron Diffraction". Journal of the American Chemical Society. 113 (14): 5149–5162. doi:10.1021/ja00014a003.
- ^ Brower, F. M.; Matzek, N. E.; Reigler, P. F.; Rinn, H. W.; Schmidt, D. L.; Snover, J. A.; Terada, K. (1976). "Preparation and Properties of Aluminum Hydride". Journal of the American Chemical Society. 98 (9): 2450–2454. doi:10.1021/ja00425a011.
- ^ Finholt, A. E.; Bond, A. C. Jr.; Schlesinger, H. I. (1947). "Lithium Aluminum Hydride, Aluminum Hydride and Lithium Gallium Hydride, and Some of their Applications in Organic and Inorganic Chemistry". Journal of the American Chemical Society. 69 (5): 1199–1203. doi:10.1021/ja01197a061.
- ^ US patent 6228338, Petrie, M. A.; Bottaro, J. C.; Schmitt, R. J.; Penwell, P. E.; Bomberger, D. C., "Preparation of Aluminum Hydride Polymorphs, Particularly Stabilized α-AlH3", issued 2001-05-08
- ^ Schmidt, D. L.; Roberts, C. B.; Reigler, P. F.; Lemanski, M. F. Jr.; Schram, E. P. (1973). Aluminum Trihydride-Diethyl Etherate: (Etherated Alane). Inorganic Syntheses. 14. pp. 47–52. doi:10.1002/9780470132456.ch10. ISBN 9780470132456.
- ^ Alpatova, N. M.; Dymova, T. N.; Kessler, Yu. M.; Osipov, O. R. (1968). "Physicochemical Properties and Structure of Complex Compounds of Aluminium Hydride". Russian Chemical Reviews. 37 (2): 99–114. Bibcode:1968RuCRv..37...99A. doi:10.1070/RC1968v037n02ABEH001617.
- ^ Semenenko, K. N.; Bulychev, B. M.; Shevlyagina, E. A. (1966). "Aluminium Hydride". Russian Chemical Reviews. 35 (9): 649–658. Bibcode:1966RuCRv..35..649S. doi:10.1070/RC1966v035n09ABEH001513.
- ^ Osipov, O. R.; Alpatova, N. M.; Kessler, Yu. M. (1966). Elektrokhimiya. 2: 984.CS1 maint: untitled periodical (link)
- ^ Jump up to: a b Zidan, R.; Garcia-Diaz, B. L.; Fewox, C. S.; Stowe, A. C.; Gray, J. R.; Harter, A. G. (2009). "Aluminium hydride: a reversible material for hydrogen storage". Chemical Communications (25): 3717–3719. doi:10.1039/B901878F. PMID 19557259. S2CID 21479330.
- ^ Jump up to: a b Martinez-Rodriguez, M. J.; Garcia-Diaz, B. L.; Teprovich, J. A.; Knight, D. A.; Zidan, R. (2012). "Advances in the electrochemical regeneration of aluminum hydride". Applied Physics A: Materials Science & Processing. 106 (25): 545–550. Bibcode:2012ApPhA.106..545M. doi:10.1007/s00339-011-6647-y. S2CID 93879202.
- ^ DE patent 1141623, Clasen, H., "Verfahren zur Herstellung von Aluminiumhydrid bzw. aluminiumwasserstoffreicher komplexer Hydride", issued 1962-12-27, assigned to Metallgesellschaft
- ^ US patent 8470156, Zidan, R., "Electrochemical process and production of novel complex hydrides", issued 2013-06-25, assigned to Savannah River Nuclear Solutions, LLC
- ^ Saitoh, H; Sakurai, Y; Machida, A; Katayama, Y; Aoki, K (2010). "In situX-ray diffraction measurement of the hydrogenation and dehydrogenation of aluminum and characterization of the recovered AlH3". Journal of Physics: Conference Series. 215 (1): 012127. Bibcode:2010JPhCS.215a2127S. doi:10.1088/1742-6596/215/1/012127. ISSN 1742-6596.
- ^ Jump up to: a b Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ^ Atwood, J. L.; Bennett, F. R.; Elms, F. M.; Jones, C.; Raston, C. L.; Robinson, K. D. (1991). "Tertiary Amine Stabilized Dialane". Journal of the American Chemical Society. 113 (21): 8183–8185. doi:10.1021/ja00021a063.
- ^ Yun, J.-H.; Kim, B.-Y.; Rhee, S.-W. (1998). "Metal-Organic Chemical Vapor Deposition of Aluminum from Dimethylethylamine Alane". Thin Solid Films. 312 (1–2): 259–263. Bibcode:1998TSF...312..259Y. doi:10.1016/S0040-6090(97)00333-7.
- ^ Galatsis, P. (2001). "Diisobutylaluminum Hydride". Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rd245. ISBN 978-0-470-84289-8.
- ^ Ayres, D. C.; Sawdaye, R. (1967). "The Stereoselective Reduction of Ketones by Aluminium Hydride". Journal of the Chemical Society B. 1967: 581–583. doi:10.1039/J29670000581.
- ^ Corey, E. J.; Cane, D. E. (1971). "Controlled Hydroxymethylation of Ketones". Journal of Organic Chemistry. 36 (20): 3070. doi:10.1021/jo00819a047.
- ^ Jorgenson, Margaret J. (July 1962). "Selective reductions with aluminum hydride". Tetrahedron Letters. 3 (13): 559–562. doi:10.1016/S0040-4039(00)76929-2.
- ^ Takano, S.; Akiyama, M.; Sato, S.; Ogasawara, K. (1983). "A Facile Cleavage of Benzylidene Acetals with Diisobutylaluminum Hydride" (pdf). Chemistry Letters. 12 (10): 1593–1596. doi:10.1246/cl.1983.1593.
- ^ Richter, W. J. (1981). "Asymmetric Synthesis at Prochiral Centers: Substituted 1,3-Dioxolanes". Journal of Organic Chemistry. 46 (25): 5119–5124. doi:10.1021/jo00338a011.
- ^ Maruoka, K.; Saito, S.; Ooi, T.; Yamamoto, H. (1991). "Selective Reduction of Methylenecycloalkane Oxides with 4-Substituted Diisobutylaluminum 2,6-Di-tert-butylphenoxides". Synlett. 1991 (4): 255–256. doi:10.1055/s-1991-20698.
- ^ Claesson, A.; Olsson, L.-I. (1979). "Allenes and Acetylenes. 22. Mechanistic Aspects of the Allene-Forming Reductions (SN2' Reaction) of Chiral Propargylic Derivatives with Hydride Reagents". Journal of the American Chemical Society. 101 (24): 7302–7311. doi:10.1021/ja00518a028.
- ^ Corey, E. J.; Katzenellenbogen, J. A.; Posner, G. H. (1967). "New Stereospecific Synthesis of Trisubstituted Olefins. Stereospecific Synthesis of Farnesol". Journal of the American Chemical Society. 89 (16): 4245–4247. doi:10.1021/ja00992a065.
- ^ Sato, F.; Sato, S.; Kodama, H.; Sato, M. (1977). "Reactions of Lithium Aluminum Hydride or Alane with Olefins Catalyzed by Titanium Tetrachloride or Zirconium Tetrachloride. A Convenient Route to Alkanes, 1-Haloalkanes and Terminal Alcohols from Alkenes". Journal of Organometallic Chemistry. 142 (1): 71–79. doi:10.1016/S0022-328X(00)91817-5.
- ^ Calabro, M. (2011). "Overview of Hybrid Propulsion". Progress in Propulsion Physics. 2: 353–374. Bibcode:2011EUCAS...2..353C. doi:10.1051/eucass/201102353. ISBN 978-2-7598-0673-7.
- ^ 2013 CFR Title 29 Volume 6 Section 1900.1200 Appendix B.12
External links[]
- Aluminium Hydride on EnvironmentalChemistry.com Chemical Database
- Hydrogen Storage from Brookhaven National Laboratory
- Aluminum Trihydride on WebElements
- Aluminium compounds
- Reducing agents
- Metal hydrides