Germane
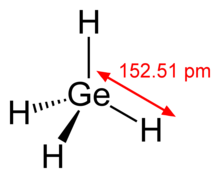
| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Germane
| |||
| Other names
Germanium tetrahydride
Germanomethane Monogermane | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.029.055 | ||
| EC Number |
| ||
| 587 | |||
| KEGG | |||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 2192 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| GeH4 | |||
| Molar mass | 76.62 g/mol | ||
| Appearance | Colorless gas | ||
| Odor | Pungent[1] | ||
| Density | 3.3 kg/m3 | ||
| Melting point | −165 °C (−265 °F; 108 K) | ||
| Boiling point | −88 °C (−126 °F; 185 K) | ||
| Low | |||
| Vapor pressure | >1 atm[1] | ||
| Viscosity | 17.21 μPa·s (theoretical estimate)[2] | ||
| Structure | |||
| Tetrahedral | |||
Dipole moment
|
0 D | ||
| Hazards | |||
| Main hazards | Toxic, flammable, may ignite spontaneously in air | ||
| Safety data sheet | ICSC 1244 | ||
| GHS labelling: | |||
   
| |||
Signal word
|
Danger | ||
| H220, H280, H302, H330 | |||
| P210, P260, P264, P270, P271, P284, P301+P312, P304+P340, P310, P320, P330, P377, P381, P403, P403+P233, P405, P410+P403, P501 | |||
| NFPA 704 (fire diamond) | 
4
4
3 | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible)
|
None[1] | ||
REL (Recommended)
|
TWA 0.2 ppm (0.6 mg/m3)[1] | ||
IDLH (Immediate danger)
|
N.D.[1] | ||
| Related compounds | |||
Related compounds
|
Methane Silane Stannane Plumbane Germyl | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Germane is the chemical compound with the formula GeH4, and the germanium analogue of methane. It is the simplest germanium hydride and one of the most useful compounds of germanium. Like the related compounds silane and methane, germane is tetrahedral. It burns in air to produce GeO2 and water. Germane is a group 14 hydride.
Occurrence[]
Germane has been detected in the atmosphere of Jupiter.[3]
Synthesis[]
Germane is typically prepared by reduction of germanium compounds, notably germanium dioxide, with hydride reagents such as sodium borohydride, potassium borohydride, lithium borohydride, lithium aluminium hydride, sodium aluminium hydride. The reaction with borohydrides is catalyzed by various acids and can be carried out in either aqueous or organic solvent. On laboratory scale, germane can be prepared by the reaction of Ge(IV) compounds with these hydride reagents.[4][5] A typical synthesis involved the reaction of Na2GeO3 with sodium borohydride.[6]
- Na2GeO3 + NaBH4 + H2O → GeH4 + 2 NaOH + NaBO2
Other methods for the synthesis of germane include electrochemical reduction and a plasma-based method.[7] The electrochemical reduction method involves applying voltage to a germanium metal cathode immersed in an aqueous electrolyte solution and an anode counter-electrode composed of a metal such as molybdenum or cadmium. In this method, germane and hydrogen gases evolve from the cathode while the anode reacts to form solid molybdenum oxide or cadmium oxides. The plasma synthesis method involves bombarding germanium metal with hydrogen atoms (H) that are generated using a high frequency plasma source to produce germane and digermane.
Reactions[]
Germane is weakly acidic. In liquid ammonia GeH4 is ionised forming NH4+ and GeH3−.[8] With alkali metals in liquid ammonia GeH4 reacts to give white crystalline MGeH3 compounds. The potassium (potassium germyl KGeH3) and rubidium compounds (rubidium germyl RbGeH3) have the sodium chloride structure implying a free rotation of the GeH3− anion, the caesium compound, CsGeH3 in contrast has the distorted sodium chloride structure of TlI.[8]
Use in semiconductor industry[]
The gas decomposes near 600K (327°C; 620°F) to germanium and hydrogen. Because of its thermal lability, germane is used in the semiconductor industry for the epitaxial growth of germanium by MOVPE or chemical beam epitaxy.[9] Organogermanium precursors (e.g. isobutylgermane, alkylgermanium trichlorides, and dimethylaminogermanium trichloride) have been examined as less hazardous liquid alternatives to germane for deposition of Ge-containing films by MOVPE.[10]
Safety[]
Germane is a highly flammable, potentially pyrophoric,[11] and a highly toxic gas. In 1970, the American Conference of Governmental Industrial Hygienists (ACGIH) published the latest changes and set the occupational exposure threshold limit value at 0.2 ppm for an 8-hour time weighted average.[12] The LC50 for rats at 1 hour of exposure is 622 ppm.[13] Inhalation or exposure may result in malaise, headache, dizziness, fainting, dyspnea, nausea, vomiting, kidney injury, and hemolytic effects.[14][15][16]
The US Department of Transportation hazard class is 2.3 Poisonous Gas.[12]
References[]
- ^ a b c d e NIOSH Pocket Guide to Chemical Hazards. "#0300". National Institute for Occupational Safety and Health (NIOSH).
- ^ Yaws, Carl L. (1997), Handbook Of Viscosity: Volume 4: Inorganic Compounds And Elements, Gulf Professional Publishing, ISBN 978-0123958501
- ^ Kunde, V.; Hanel, R.; Maguire, W.; Gautier, D.; Baluteau, J. P.; Marten, A.; Chedin, A.; Husson, N.; Scott, N. (1982). "The tropospheric gas composition of Jupiter's north equatorial belt (NH3, PH3, CH3D, GeH4, H2O) and the Jovian D/H isotopic ratio". Astrophysical Journal. 263: 443–467. Bibcode:1982ApJ...263..443K. doi:10.1086/160516.
- ^ W. L. Jolly "Preparation of the volatile hydrides of Groups IVA and VA by means of aqueous hydroborate" Journal of the American Chemical Society 1961, volume 83, pp. 335-7.
- ^ US Patent 4,668,502
- ^ Girolami, G. S.; Rauchfuss, T. B.; Angelici, R. J. (1999). Synthesis and Technique in Inorganic Chemistry. Mill Valley, CA: University Science Books.
- ^ US Patent 7,087,102 (2006)
- ^ a b Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ^ Venkatasubramanian, R.; Pickett, R. T.; Timmons, M. L. (1989). "Epitaxy of germanium using germane in the presence of tetramethylgermanium". Journal of Applied Physics. 66 (11): 5662–5664. Bibcode:1989JAP....66.5662V. doi:10.1063/1.343633.
- ^ Woelk, E.; Shenai-Khatkhate, D. V.; DiCarlo, R. L. Jr.; Amamchyan, A.; Power, M. B.; Lamare, B.; Beaudoin, G.; Sagnes, I. (2006). "Designing Novel Organogermanium MOVPE Precursors for High-purity Germanium Films". Journal of Crystal Growth. 287 (2): 684–687. Bibcode:2006JCrGr.287..684W. doi:10.1016/j.jcrysgro.2005.10.094.
- ^ Brauer, 1963, Vol.1, 715
- ^ a b Praxair MSDS accessed Sep. 2011
- ^ NIOSH Germane Registry of Toxic Effects of Chemical Substances (RTECS)accessed Sep. 2011
- ^ Gus'kova, E. I. (1974). "K toksikologii Gidrida Germaniia" [Toxicology of germanium hydride]. Gigiena Truda I Professionalnye Zabolevaniia (in Russian). 18 (2): 56–57. PMID 4839911.
- ^ US EPA Germane
- ^ Paneth, F.; Joachimoglu, G. (1924). "Über die pharmakologischen Eigenschaften des Zinnwasserstoffs und Germaniumwasserstoffs" [About the pharmacological characteristics of tin hydride and germanium hydride]. Berichte der Deutschen Chemischen Gesellschaft (in German). 57 (10): 1925–1930. doi:10.1002/cber.19240571027.
External links[]
| Look up germane in Wiktionary, the free dictionary. |
- Metaloids (manufacturer) datasheet
- Arkonic Specialty Gases China (manufacturer) datasheet
- Licensintorg Russia (process technology sale)
- Honjo Chemical Japan (manufacturer)
- Praxair datasheet
- Air liquide gas encyclopedia entry
- CDC - NIOSH Pocket Guide to Chemical Hazards
- Voltaix (manufacturer) datasheet
- Foshan Huate Gas Co.,Ltd. (manufacturer)
- Horst Technologies, Russia (manufacturer)
- Germanium compounds
- Metal hydrides
- Industrial gases

