Tzanck test
| Tzanck test | |
|---|---|
 A positive Tzanck test, showing three multinucleated giant cells ("Tzanck cells") in center. | |
| Purpose | diagnosis of varicella-zoster virus and herpes |
In dermatopathology, the Tzanck test, also Tzanck smear, is scraping of an ulcer base to look for Tzanck cells. It is sometimes also called the chickenpox skin test and the herpes skin test. It is a simple, low-cost, and rapid office based test.[1]
Tzanck cells (acantholytic cells) are found in:
- Herpes simplex[2]
- Varicella and herpes zoster
- Pemphigus vulgaris
- Cytomegalovirus
Arnault Tzanck did the first cytological examinations in order to diagnose skin diseases.[3] To diagnose pemphigus, he identified acantholytic cells, and to diagnose of herpetic infections he identified multinucleated giant cells and acantholytic cells. He extended his cytologic findings to certain skin tumors as well.
Even though cytological examination can provide rapid and reliable diagnosis for many skin diseases, its use is limited to a few diseases. In endemic regions, Tzanck test is used to diagnose leishmaniasis and leprosy. For other regions, Tzanck test is mainly used to diagnose pemphigus and herpetic infections. Some clinics use biopsies even for herpetic infections.[4] This is because the advantages of this test are not well known, and the main textbooks of dermatopathology do not include dedicated sections for cytology or Tzanck smear.[5] A deep learning model called TzanckNet has been developed to lower the experience barrier needed to use this test.[6]
Procedure[]
- Unroof vesicle and scrape base w/ sterile №15 scalpel blade
- Smear onto a clean glass slide
- Fix w/ gentle heat or air dry
- Fix w/ MeOH (Methanol)
- Stain w/ Giemsa, methylene blue or Wright’s stain.
- Microscopic examination using an oil immersion lens. (Look for multinucleated giant cells)[7]
A modified test can be performed using proprietary agents which requires fewer steps and allows the sample to be fixed quicker.
Cytologic findings[]
For microscopic evaluation, samples are first scanned with low magnification objectives (X4 and X10) and then examined in detail with the high magnification objective (X100). The X4 objectives are used to select the areas to investigate in detail and to detect some ectoparasites, but the basis of the cytological diagnostic process is the X10 objective. With X10 magnification, the individual characteristics of the cells, the relationship of the cells to each other and the presence of some infection and infestation agents are evaluated. For this reason, most of the cytological examination is spent at this magnification, and most samples are diagnosed at this magnification. The key cytological findings that are observed at low magnification or, in other words, should be investigated according to the clinical characteristics of the patient are as follows: acantholytic cells, tadpole cells, granulomatous inflammation, infectious agents and increases in specific cells.
Major indications, cytologic findings and diagnostic value of Tzanck smear test[]
| Diseases | Cytologic findings | Diagnostic value | ||
| I. Cutaneous infections | ||||
| Bacterial infections | ||||
| Bullous impetigo | Dyskeratotic acantholytic cells, abundant neutrophils and clusters of cocci | 92% sensitive and 100% specific | ||
| Staphylococcal scalded skin syndrome (SSSS) | Dyskeratotic acantholytic cells, absence of abundant neutrophils and cocci | A Tzanck smear may be a rapid test to distinguish toxic epidermal necrolysis from SSSS | ||
| Mycobacterial infections | Negative images of mycobacteriae, acid-fast bacilli | Maximum acid-fast bacilli positivity (94%) in cases showing caseation necrosis with or without granulomas | ||
| Bacillary angiomatosis | Clumps of coccobacilli of B Henselae in Warthin-Starry-stained smears | |||
| Fungal infections | ||||
| Dermatophytic infections | Hyphae and spores | |||
| Candidiasis | Pseudohyphae and spores | Pseudohyphae and spores in 90% of patients with candida folliculitis | ||
| Aspergillosis | Septate hyphae with 45-degree angle branching and/or aspergillus heads | |||
| Blastomycosis | Broad-based budding spores | 57.7 - 93% sensitive | ||
| Sporotrichosis | Spherical, oval or cigar-shaped yeasts and asteroid bodies | 84.9% sensitive and 57.9% specific | ||
| Viral infections | ||||
| Herpetic infections | Acantholytic cells, multinucleated giant cells and eosinophilic inclusion bodies | 53.1 - 86% sensitive and 100% specific | ||
| Hand, foot and mouth disease | Syncytial nuclei, absence of acantholytic cells1 | 72% sensitive and 100% specific | ||
| Human papillomavirus infections | Koilocytes | 75% sensitive and 100% specific | ||
| Molluscum contagiosum | Intracytoplasmic inclusion bodies (“Henderson-Patterson’s bodies”) | |||
| Milker’s nodule and orf | Intracytoplasmic inclusion bodies (“Guarnieri’s bodies”) | |||
| Parasitic infections | ||||
| Leishmaniasis | Ellipsoid-shaped Leishman-Donovan bodie | 30 - 82.6% sensitive and 100% specific | ||
| Demodicosis | More than 5 Demodex mites/cm2 | The diagnostic value of cytology for diagnosing Demodex folliculitis is higher than that of histopathology. The sensitivity of histopathology is 60%, whereas that of cytology is 93.3% | ||
| Cutaneous amoebiasis | Trophozoites of Entamoeba histolytica | Direct specimens or PAS and acid phosphatase-stained specimens in cases with doubtful direct specimens show trophozoites of Entamoeba histolytica | ||
| II. Immunobullous disorders | ||||
| Pemphigus | Acantholytic cells with direct immunofluorescence positivity | With the use of direct immunofluorescence examination, the specificity of cytology for diagnosing pemphigus can be increased from 43% to 100%. | ||
| Erythema multiforme, Toxic epidermal necrolysis | Apoptotic and necrotic cells, absence of acantholytic cells | A Tzanck smear may be a rapid test to distinguish toxic epidermal necrolysis from SSSS | ||
| III. Genodermatoses | ||||
| Hailey-Hailey disease | Acantholytic cells without direct immunofluorescence positivity | Direct immunofluorescence examination should be made for differentiation from pemphigus. | ||
| Darier’s disease | Acantholytic cells, corps ronds, grains | |||
| IV. Spongiotic dermatitis | Presence of more than 10 tadpole cells at x100 magnification | |||
| Contact dermatitis | Tadpole cells and lymphocytes | 83% sensitive and 100% specific | ||
| V. Granulomatous diseases | Granuloma formation and multinucleated giant cells | The main purpose of cytological examination in granulomatous dermatitis is to detect infectious agents. Foreign body materials are very specific for foreign body granuloma. | ||
| Granuloma annulare | Palisading granuloma and mucin | |||
| Necrobiosis lipoidica | Palisading granuloma and necrobiotic materials | |||
| Foreign-body granuloma | Foreign body | |||
| Juvenile xanthogranuloma | Touton type giant cells and foamy cells | |||
| VI. Tumoral lesions | ||||
| Benign tumoral lesions | ||||
| Mastocytoma | Abundant mast cells | Tzanck smear test is useful for rapid diagnosis of mastocytoma in children | ||
| Sebaceous hyperplasia | Clusters of sebocytes | |||
| Seborrheic keratosis | Hyperkeratosis and horny cysts | 87.5% sensitive and 80.8% specific | ||
| Melanocytic nevi | Dermal and epidermal type nevoid cells | 87.5% sensitive and 100% specific | ||
| Eruptive vellus hair cysts | Abundant vellus hairs | |||
| Malignant tumoral lesions | Cellular atypia including mitosis, poikilokaryosis, poikilocytosis, nuclear contour irregularity, prominent nucleoli and nuclear molding | |||
| Basal cell carcinoma | Clusters of basaloid cells | The most common reason to use cytological examination in malignant tumoral diseases is to distinguish basal cell carcinoma from other tumors, such as squamous cell carcinoma and melanoma. Tzanck smear test is 97% sensitivity and 86% specificity for the diagnosis of basal cell carcinoma. | ||
| Squamous cell carcinoma | Cytologic atypia of keratinocytes | |||
| Melanoma | Atypical melanocytes | |||
| Lymphoma | Atypical lymphocytes | |||
| Paget’s disease | Isolated or clusters of Paget’s cells | |||
| Kaposi’s sarcoma | Cigar-shaped spindle cells | |||
Tzanck smear examples[]

Acantholytic cell and multinucleated giant cell on a smear taken from a vesicular lesion of herpes simplex infection (May-Grünwald Giemsa x1000).

Extracellular and intracellular (macrophages) leishmania parasites in a patient with cutaneous leishmaniasis (May-Grünwald Giemsa x1000).

Acantholytic cells on a smear taken from a patient with pemphigus foliaceus (May-Grünwald Giemsa x1000).
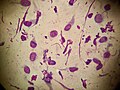
Tadpole cells on a smear taken from a patient with allergic contact dermatitis (May-Grünwald Giemsa x1000).

A cluster of basaloid cells on a smear taken from a patient with basal cell carcinoma (May-Grünwald Giemsa x1000).
References[]
- ^ Durdu, Murat (2019). Cutaneous Cytology and Tzanck Smear Test. Springer International Publishing. ISBN 978-3-030-10721-5.
- ^ Folkers E, Oranje AP, Duivenvoorden JN, van der Veen JP, Rijlaarsdam JU, Emsbroek JA (August 1988). "Tzanck smear in diagnosing genital herpes". Genitourinary Medicine. 64 (4): 249–54. doi:10.1136/sti.64.4.249. PMC 1194227. PMID 3169755.
- ^ Tzanck, Arnault (1947). "Le cyto-diagnostic immédiat en dermatologie". Presse Médicale.
- ^ Horn, Thomas D. (December 2008). "Commentary: heading the wrong way: the disappearing Tzanck smear". Journal of the American Academy of Dermatology. 59 (6): 965–966. doi:10.1016/j.jaad.2008.08.025. ISSN 1097-6787. PMID 18929430.
- ^ Kelly, Brent; Shimoni, Tally (2009-06-01). "Reintroducing the Tzanck Smear". American Journal of Clinical Dermatology. 10 (3): 141–152. doi:10.2165/00128071-200910030-00001. ISSN 1179-1888. PMID 19354329.
- ^ Noyan, Mehmet Alican; Durdu, Murat; Eskiocak, Ali Haydar (2020-10-27). "TzanckNet: A convolutional neural network to identify cells in the cytology of erosive-vesiculobullous diseases". Scientific Reports. 10 (1): 18314. doi:10.1038/s41598-020-75546-z. PMC 7591506. PMID 33110197.
- ^ Pettit, Normal Microbiota of the Skin, ATSU School of Osteopathic Medicine Arizona, Lecture Slides. Jan 2013.
External links[]
- Tzanck test - medlineplus.org.
- Definition of Tzanck test - medterms.com.
- Medical tests
- Pathology
- Chickenpox




